Thames & Kosmos Chemistry C500 (Version 2.0) handleiding
Handleiding
Je bekijkt pagina 14 van 52
Flame out!
YOU WILL NEED
→ 2 test tubes
→ measuring spoon
→ dropper pipette
→ sodium carbonate
→ tartaric acid
→ matches
→ cup of water
→ paper
→ scissors
HERE’S HOW
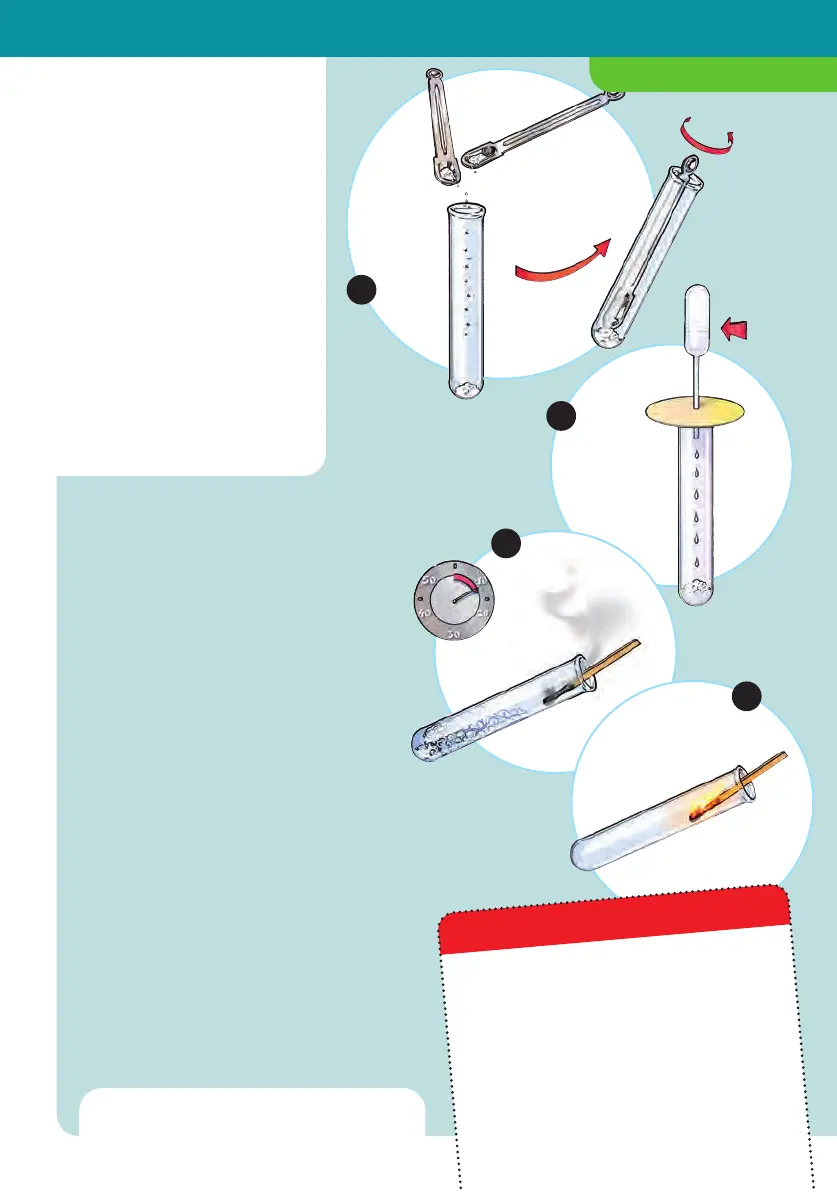
1. In a dry test tube, mix 1 large spoonful
of sodium carbonate with 1 large
spoonful of tartaric acid.
2. Cut a circular shape out of a piece of
paper. Punch a hole in the center with
the tip of a ballpoint pen and insert the
pipette into it. Fill the pipette with
water and set it along with its “collar”
onto the test tube. Slowly drip water
into the test tube.
3. Wait about 10 to 15 seconds for the
foam to subside a little. Remove the
pipette. Now hold a burning match in
the test tube.
Does it keep burning?
4. Perform the same “match test” with an
unused, fresh test tube.
What happens?
For the match to burn, it needs oxygen,
which is contained in the air. The carbon
dioxide produced in the first test tube,
however, is heavier than air and
therefore pushes the air out of the test
tube. So the match flame is lacking in
oxygen, and it goes out.
3
1
4
2
1
x
SODIUM
CARBONATE
1
x
TARTARIC
ACID
WATER
Safety Note:
For sodium carbonate and tartaric acid, note the
“Information about hazardous substances” on page 7!
→
WHAT’S HAPPENING?
12
EXPERIMENT 3
Bekijk gratis de handleiding van Thames & Kosmos Chemistry C500 (Version 2.0), stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Thames & Kosmos |
| Model | Chemistry C500 (Version 2.0) |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 25520 MB |







