Medisana TT 250 handleiding
Handleiding
Je bekijkt pagina 2 van 26
DE/GB 88352 TT 250 20-Nov-2024 Ver. 1.0
GB Instruction manual
Menstrual Pain Reliever with TENS & Heat TT 250
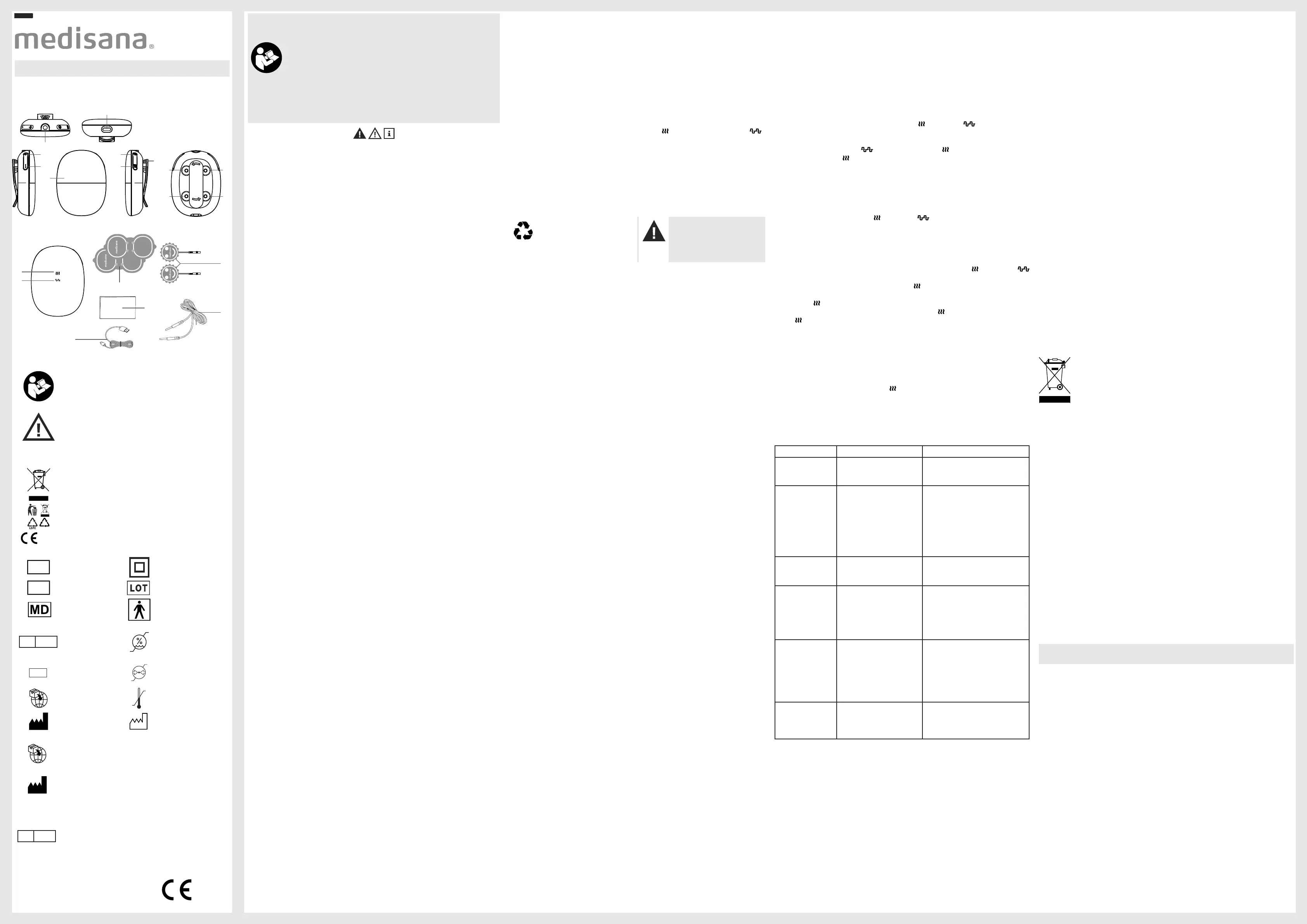
Device and controls
Figure 1:
Figure 2:
Explanation of symbols
This instruction manual belongs to this device. The
instruction manual includes important information on
the initial start-up and handling. Read this instruction
manual completely. Failure to follow these instructions
may result in serious injury or damage to the device.
CAUTION
General warning sign. Please refer to the instruction
before using.
IP22
IP22
The device is protected against splashing water. Wa-
ter splashed against the enclosure from any direction
VKDOOKDYHQRKDUPIXOHႇHFWV
Disposal only according to the “WEEE” Waste Electri-
cal and Electronic Equipment EC Directive.
04
PAP
20
Recycling symbols/codes: These are used to provide
information about the material and its proper use and
recycling.
1639
Complies with the European Medical Device Regula-
WLRQ(81RWL¿HG%RG\LV6*6%HOJLXP
NV
REF
Catalogue Num-
ber
Electric shock pro-
tection type: Class II
equipment
S
N
Serial Number Batch code
Medical Device
'HYLFHFODVVL¿FDWLRQW\SH
BF applied part
EC REP
Authorized
representative
in the European
Community / Eu-
ropean Union
Humidity range
UDI
Unique Device
,GHQWL¿HU
Ambient pressure
limitation
Importer Temperature range
Manufacturer Date of manufacture
medisana GmbH,
Carl-Schurz-Str. 2, 41460 NEUSS,
GERMANY
Famidoc Technology Co., Ltd.
NO.212 Yilong Road, Changan Town, Dongguan,
Guangdong Province,
523853, P.R.China.
Tel.: +86-769-89272488 Fax: +86-769-89272498
Website: www.famidoc.com
EC REP
Shanghai International Holding Corp. GmbH
(Europe)
(LႇHVWUDH+DPEXUJ*(50$1<
GB IMPORTANT INFORMATION!
KEEP IN A SAFE PLACE!
Please read this instruction manual care-
fully, in particular the safety instructions,
before using the device. Keep the in-
struction manual in a safe place for later
reference. If you pass the device on to a
third party, this instruction manual must
remain with the device.
WARNING
• This therapeutic apparatus is not recommended to use in the following cases:
ʊ 1) Do not use this device if the patient has any implantable electronic or metal
equipment (e.g. heart pacemaker).
ʊ 2) Epilepsy.
ʊ 3) Severe lower extremity arterial circulation problems.
ʊ 4) Abdominal or inguinal hernia site.
ʊ 5) Do not use this device if you have a heart disease without consulting a
doctor.
ʊ 6) Being pregnant.
ʊ 7) Cancer.
ʊ 8) Having Fever.
ʊ 9) Do not use therapeutic instruments in high humidity environments, such as
bathing or showering in a bathroom bathtub.
ʊ 10) Do not use after drinking.
ʊ 11) Do not use Low-frequency therapeutic instruments when using high fre-
quency surgical instruments.
ʊ 12) Don’t use close to the genitals.
ʊ 13) Recommendation: Do not use low-frequency therapeutic instruments near
shortwave or microwave therapeutic equipment, where the output of the low
frequency therapeutic instrument used is unstable. Keep a safe distance – at
least 1 m – to such devices.
ʊ 14) The high frequency surgical equipment and stimulator. When connected
to a patient, burns may occur at the stimulator electrode and may damage the
stimulator.
ʊ 15) Abnormal feeling of the patient having medical treatment.
ʊ 16) Patients with cardiac and neurological abnormalities.
ʊ 17) Patients with body temperature exceeding 38°C.
ʊ 18) Patients with infectious diseases.
ʊ 19) Patients who can not express their will.
ʊ 20) Do not use this product while charging.
• Note:
ʊ 1) Make sure you are always aware of all warning items.
ʊ 2) This product should not be used in the throat and mouth. This may result
in severe throat muscle spasms and muscle contraction,which will likely block
the airway and cause dyspnea.
ʊ 3) This product should not be used in front of the chest, as the current intro-
duced into the heart can lead to cardiac arrhythmia and cardiac arrest.
ʊ 7KLVSURGXFWVKRXOGQRWEHXVHGRQWKHXQDWWHQGHGSDWLHQWVRUWKRVHVXႇHU
from emotional distress, dementia, and with low IQ.
ʊ 5) Use guidance has been listed in details; any improper use may lead to
danger.
ʊ 6) Do not use this device for any un-diagnosed pain symptoms. Do consult a
doctor for diagnosis before use.
ʊ 7) Electrodes can not be placed in areas that may cause current to pass
through the brain (i.e. one-sided electrodes can not be placed on the front
and back of the head at the same time, or relative to the left and right sides
of the head).
ʊ 3DWLHQWVVXႇHULQJIURPKHDUWGLVHDVHFDQFHURURWKHUKHDOWKWKUHDWHQLQJ
illnesses should only use this device after consulting a doctor.
ʊ 9) If the intensity of stimulation makes you feel uncomfortable, reduce the
intensity of stimulation to a comfortable level. If the problem remains, please
contact your doctor.
ʊ 10) Do not use this equipment in the rooms where aerosol (spray) and pure
oxygen are used.
ʊ 3OHDVHGRQRWXVHWKLVHTXLSPHQWQHDUDQ\KLJKO\ÀDPPDEOHVXEVWDQFHV
gases or explosives.
ʊ 12) Please check the cable and related connections are in good condition
before each use.
ʊ 7XUQRႇWKHGHYLFHEHIRUHXVLQJRUUHPRYLQJWKHHOHFWURGHSDG
ʊ 14) This product can only be used with electrode wires and electrodes recom-
mended by the manufacturer.
• Consult your doctor before using the device, especially when:
ʊ $FXWHGLVHDVHHVSHFLDOO\LQSDWLHQWVVXVSHFWHGRUVXႇHULQJIURPK\SHUWHQ-
sion, thrombosis or malignant mass.
ʊ Patients with various skin diseases.
ʊ Patients with chronic pain disorders (e.g. metabolic disorders) with reduced
pain.
ʊ Patients taking medication.
ʊ
Adverse reactions
ʊ Skin allergies caused by electrode sheet pad gels and electrode irritation
burns are potential adverse reactions. If allergic symptom appears on skin,
please discontinue the treatment and consult your doctor.
ʊ If the intensity of stimulation makes you feel uncomfortable, reduce the inten-
sity of stimulation to a comfortable level. If the problem persists, contact your
doctor.
• Do not use this product while charging.
• ,OOXVWUDWLRQVLQWKLVPDQXDODUHHႇHFWGUDZLQJVIRUUHIHUHQFHRQO\*RRGVLQNLQG
prevail.
• Thanks for using our product. Please pay attention to the instructions and read
them carefully before using this product.
• Please ensure the compliance of selected adapters to relevant safety medical
OHYHODQGVDIHW\FHUWL¿FDWLRQUHTXLUHPHQWVH[DPSOH,(&
• After connection, it will be charged automatically.
Working Principle
TENS, or transcutaneous electrical nerve stimulation, refers to the electrical stimu-
ODWLRQRIQHUYHVWKURXJKWKHVNLQ7(16LVDQHႇHFWLYHQRQSKDUPDFRORJLFDOPHWKRG
IRUWUHDWLQJGLႇHUHQWW\SHVRISDLQIURPDYDULHW\RIFDXVHV
7KHSDLQUHOLHYLQJRUSDLQVXSSUHVVLQJHႇHFWLVDFKLHYHGE\LQKLELWLQJWKHWUDQVIHU
RISDLQWRQHUYH¿EUHVDQGE\LQFUHDVLQJWKHVHFUHWLRQRIHQGRUSKLQVLQWKHERG\
DVWKHLUHႇHFWRQWKHFHQWUDOQHUYRXVV\VWHPUHGXFHVWKHVHQVDWLRQRISDLQ7KH
PHWKRGLVVFLHQWL¿FDOO\VXEVWDQWLDWHGDQGDSSURYHGDVDIRUPRIPHGLFDOWUHDWPHQW
Product structure and composition
Intensity [+] button Intensity [-] button Display screen
2Q2ႇEXWWRQ Heat button
Connection channel for electrode cable
Type-C charging interface
Magnetic fasteners Heat icon TENS icon
2 × TENS & Heating pads 1 × this instruction manual
1 × Type-C charging cable 2 × Electrode pads
1 × Electrode wire Detachable belt clip
Notes:
• This product does not come with an adapter. Please purchase separately.
• If you have any problems with the adapters, consult your dealer at the place of
purchase.
• If you notice any transport damage when unpacking, please contact your dealer
immediately.
The packaging can be reused
or recycled. Please dispose
properly of any packaging
material no longer required.
WARNING
Please ensure that the poly-
thene packing is kept away
from the reach of children!
5LVNRIVXႇRFDWLRQ
Intended use
The device is intended to temporary pain relief.
• On people
• For external use
• For home and private use
• For muscle pains relief
• For menstrual pain relieve
• For body relaxation
Contraindications:
• This device should not be used when cancerous lesions are present in the treat-
ment area.
• 6WLPXODWLRQVKRXOGQRWEHDSSOLHGRYHUVZROOHQLQIHFWHGLQÀDPHGDUHDVRUVNLQ
eruptions (e.g. phlebitis, thrombophlebitis, varicose veins, etc.).
• Safety has not been established for the use of therapeutic electrical stimulation
during pregnancy.
• 'RQRWXVHWKLVGHYLFHLI\RXKDYHDFDUGLDFSDFHPDNHULPSODQWHGGH¿EULOODWRU
or other implanted metallic or electronic device. Such use could cause electric
shock, burns, electrical interference, or death.
• Epilepsy
• Serious arterial circulatory problems in the lower limbs
• Abdominal or inguinal hernia
• Not suitable for use during pregnancy or labor women
• Do not use this device when pain syndromes are undiagnosed. Use only after
origin of pain has been diagnosed.
• If you have heart disease without consulting your physician.
• Do not use on scarred areas following surgery for at least 10 months after the
operation.
• This device should not be used over poorly enervated areas.
• High fever
• Electrode placements must be avoided that apply current to the carotid sinus
region (anterior neck) or transcerebrally (through the head).
Intended patient population
For TENS mode: To be used for temporary relief of pain associated with sore and
aching muscles due to strain from exercise or normal household work activities.
For menstrual pain: Many women experience abdominal cramps and severe period
SDLQEHIRUHDQGGXULQJWKHLUSHULRGDQGPD\DOVRVXႇHUIURPLOOQHVVHVZKLFKFDXVH
pain in the lower abdomen, such as endometriosis. These pains are often so strong
that women seek immediate pain relief. This device is a self-adhesive TENS device
with heat function to provide pain relief for menstrual discomfort and pain caused
by endometriosis. The TENS technology used makes it possible to treat pain in a
WDUJHWHGPDQQHU7KHGHYLFHIHDWXUHVWRWDOLQWHQVLW\OHYHOVIRUGLႇHUHQWGHPDQGV
It is very a safe and easy way of relieving pain.
Intended users: Users must be 18 years or older and be able to control the device
and understand the instructions. Professional users are also suitable.
Use environment, user and patient group: Clinics or home use.
Application
Operation steps
• Connect the port of the Electrode pads to the wire terminals.
• Insert the plug of the electrode cable into the channel provided in the main
device.
• Connect the magnetic clamps from the main unit and the TENS & Heat Pad .
• Attach all pads to the treatment area.
• Press «ON/OFF» button and select the program.
Installation and use [detailed operation description]
• Place the TENS & heat pad and/or the electrode pad on the areas to be
treated.
• Press the «ON/OFF» button to turn on the device for standby mode (the de-
vice emits a “beep” with both icons heating
and TENS ÀDVKLQJ,IWKHUH
LVQRRSHUDWLRQIRUDSSUR[VHFRQGVWKHGHYLFHZLOOVZLWFKRႇDXWRPDWLFDOO\
• During standby mode, press [+] button to start the TENS function (the device
emits a “beep”, while the
icon stays on and the LFRQWXUQVRႇ,IKHDWLQJLV
needed, press the
icon for activation (the buzzer prompts «beep beep» and
TENS and heat functions are both active at the same time). If there is no opera-
tion for 30 seconds, the device will be locked automatically. Press the [-] button
to unlock.
• During TENS mode, press [+] button to increase intensity - there are 20 lev-
els available. Press the [-] button to lower intensity. When intensity has been
lowered to level 0, there is no more TENS output and the device will return to
standby mode (icons heating
and TENS ÀDVKLQJ,IWKHUHLVQRRSHUDWLRQ
IRUDSSUR[VHFRQGVWKHGHYLFHZLOOVZLWFKRႇDXWRPDWLFDOO\5HPDUN7KHUH
will always be a «beep» sound when pressing buttons or . If you continue to
press one of the buttons after max. or min. level has already been reached, the
device emits «beep beep beep beep».
• 'HIDXOWWUHDWPHQWWLPHPLQXWHV:KHQWUHDWPHQWLV¿QLVKHGWKHGHYLFHDX-
tomatically returns to standby mode with both icons heating
and TENS
ÀDVKLQJ
• In process of TENS treatment, press the
icon to start heating (device emits
©EHHSEHHSª7KHGHIDXOWKHDWLQJWHPSHUDWXUHLV&7RWXUQLWRႇDJDLQMXVW
press the
icon again (device emits “beep”) and the heating icon will extinguish.
• During heating treatment without TENS, only the
icon stays on. If you press
the
icon, heating will stop and device returns to standby mode.
• To terminate treatment, press «ON/OFF» button .
• When the device detects unproper electrode pads attachment under the TENS
mode, the buzzer will emit << beep beep beep beep>> after 2 seconds and then
turn to standby mode. If this happens in the heating mode, the device will not be
DႇHFWHGDQGLWZLOOFRQWLQXHWKHKHDWIXQFWLRQQRUPDOO\XQWLOLWLVFRPSOHWHGDIWHU
30 minutes. After that device returns to standby mode.
• When charging the product, it will switch from standby/operational mode to
charging mode. The heating icon
ZLOOÀDVKGXULQJFKDUJLQJSURFHVVDQGZLOO
constantly stay on after the device is fully charged.
• When the device has low power, the buzzer will emit <<beep beep beep beep>>
DQGWKHQGHYLFHZLOOVZLWFKRႇDXWRPDWLFDOO\
• $IWHU¿QLVKLQJWKHWUHDWPHQWSOHDVHFDUHIXOO\UHPRYHWKHHOHFWURGHSDGVIURPWKH
VNLQ5HSDVWHWKHSURWHFWLYH¿OPRYHUWKHJHOSDGIRUSURWHFWLRQ
Anomalies (Troubleshooting)
Issues Possible causes Solution
Unable to boot 1.The battery is empty
2. Malfunction
1. Charge the device
2. Call for after-sales mainte-
nance.
Automatic Stop-
ping
No human body detected 1. The electrode pad was not
attached well.
2. The skin is too dry. Wet the
skin and use it again.
3. Electrode pad is abnormal.
Replace electrode pad.
4. Electrode line is abnormal.
Replace electrode line.
Key failure The machine is locked
Press the [-] button to unlock
(see operating instructions for
details).
Weak electrical
stimulation signal
Electrode issues:
1. Air drying or contami-
nation.
2. Position problems or
wire problems.
3. Wear & tear damage.
Replace / Reconnect
Feeling unwell 1. Too much intensity
2. Too close distance
between electrodes
3. Damage to electrodes
or conductors
4. The electrode area is
too small
1. Reduce strength
2. Reorient electrode position
3. Replacement
4. Replace the electrode pad
with an area size of not less
than 16 cm (4 × 4 cm).
Stimulus failure Improper placement of
electrodes and unknown
applications
Replacement of electrodes and
follow the operating instructions.
Contact your doctor if you can-
not solve the problem.
After the treatment is completed / Cleaning
Clean the electrode pads and the main unit carefully if they have become dirty or
soiled. Therefore, wipe the surfaces with a soft, slightly moistened (use water or a
soft, neutral detergent) cloth. Let it dry thoroughly before using it again.
7HFKQLFDO6SHFL¿FDWLRQV
Name and model: medisana Menstrual Pain Reliever with TENS & Heat
TT 250 (model FDES106)
Charging time: KRXUV
Output current:
max. 70 mA
Output voltage (peak): max. 35V ±20%
Power supply: 3,7V lithium battery (5V/1A adapter)
Load resistance:
Product service life 5 years
Operating environ-
ment:
Temperature: 41°F – 104°F / 5°C – 40°C, Humidity:
15%RH – 90%RH, no condensation, Atmospheric pres-
sure: 70kPa – 106kPa
Storage environment: Temperature: 14°F – 122°F / -10°C – 50°C, Humidity:
15%RH – 95%RH, Atmospheric pressure: 70kPa –
106kPa
Type of waveform: TENS / Pulse type: Two-way symmetrical square wave
Number of channels: 2 channels
Intensity: 0– 20 (adjustable)
Output frequency: 8– 100Hz ±10%
Output pulse width: 75– 250μs ±10%
Treatment time: 30 minutes
Electric shock: Class II equipment
Applicable part: BF Application section
Water protection
grade:
IP 22
Heating temperature: 43°C ±2°C
Size of heating pads: 181 × 86,33 mm ±0,5 mm
Dimensions
(L×W×H):
approx. 52 × 69 × 24 mm
Weight: approx. 41 g
Article number: 88352
EAN number: 4015588 88352 1
Replacement parts (available separately – not part of the TT 250 delivery):
• Set with 2 x TENS & heat pads, art. 88353 / EAN 4015588 88353 8
• Set with 4 electrode pads, art. 88354 / EAN 401558888354 5
• Electrode cable, Art. 88355 / EAN 4015588 88355 2
Disposal — Waste Electrical and Electronic Equipment (WEEE)
This product is subject to European Directive 2012/19/EU on waste
electrical and electronic equipment and is marked accordingly. Never
dispose of electronic devices with household waste. Please seek out in-
formation about the local regulations with regard to the correct disposal
of electrical and electronic products. Correct disposal helps to protect
the environment and human health.
All users are obliged to hand in all electrical or electronic devices, regardless of
whether or not they contain toxic substances, at a municipal or commercial collec-
tion point so that they can be disposed of in an environmentally acceptable manner.
Consult your local authority or your supplier for information about disposal.
Warranty and repair terms
Your statutory warranty rights are not restricted by our guarantee below. Please
contact your dealer or the service centre in case of a claim under the warranty. If
you have to return the unit, please enclose a copy of your receipt and state what
the defect is.
The following warranty terms apply:
1. The warranty period for medisana products is three years from date of purchase.
In case of a warranty claim, the date of purchase has to be proven by means of
the sales receipt or invoice.
2. Defects in material or workmanship will be removed free of charge within the
warranty period.
3. Repairs under warranty do not extend the warranty period either for the unit or
for the replacement parts.
4. The following is excluded under the warranty:
a. All damage which has arisen due to improper treatment, e.g. non-obser-
vance of the user instructions.
b. All damage which is due to repairs or tampering by the customer or unau-
thorised third parties.
c. Damage which has arisen during transport from the manufacturer to the
consumer or during transport to the service centre.
d. Applied parts which are subject to normal wear and tear.
5. Liability for direct or indirect consequential losses caused by the unit are exclud-
ed even if the damage to the unit is accepted as a warranty claim.
In accordance with our policy of continual product improvement, we
reserve the right to make technical and visual changes without notice.
The complete instructions for use are available for download at
https://docs.medisana.com/88352
.
Information about service can be found here:
https://www.medisana.com/servicepartners
1639
Bekijk gratis de handleiding van Medisana TT 250, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Medisana |
| Model | TT 250 |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 16228 MB |







