Medisana IN 700 handleiding
Handleiding
Je bekijkt pagina 2 van 8
DE/GB
Device and controls
1
Nebulizer head
2
Cover
3
Lock
4
Medicine cup
5
Aerosol head
6
Electrodes
7
Power indicator
8
ON/OFF button
9
Main unit
0
Disassembly button
q
Charging cable port
w
Mouthpiece
e
Adult mask
r
Child mask
t
USB-C cable
1
2
3
4
5
6
7
8
9
0
q
w e
r
t
This instruction manual belongs to this device.
It contains important information about starting up and
operation. Read the instruction manual thoroughly.
Non-observance of these instructions can result in serious
injury or damage to the device.
WARNING
These warning notes must be observed to prevent any
injury to the user.
CAUTION
These notes must be observed to prevent any
damage to the device.
NOTE
These notes give you useful additional information
on the installation or operation.
Device classication:
type BF
Authorized EU representative
LOT number Serial number
Manufacturer Date of manufacture
Medical device Temperature range
Ambient pressure
limitation
Humidity range
SN
Explanation of symbols
CE marking in confor-
mity with EC directive
93/42/EEC
CAUTION /
注意!查阅随机文件
Type BF applied part /
BF型应用设备
Read the instructions for use /
请阅读说明书
Device in protection class 2 /
二类设备
Manufacturer / 制造商
Keep dry / 保持干燥 / 怕雨
WEEE
S
N
Serial number
Permissible storage and transport
temperature and humidity
Storage/Transport
Operation
Permissible operating temperature
and humidity
Medical device
Distributor
Date of manufacture
Do not use outdoors
(indoor use only)
Single patient multiple use
PA
2020F213-44
中国GCC血压计计量证号
REF
Reference number
LOT
Batch number
I
ON
OFF
Dispose of packaging in an
environmentally friendly manner
70°C 95%
-25°C
Storage/Transport
10% 700hPa
1060hPa
10°C
40°C
95%
700hPa
1060hPa
Operating
10%
Importer
CAUTION /
注意!查阅随机文件
Type BF applied part /
BF型应用设备
Read the instructions for use /
请阅读说明书
Device in protection class 2 /
二类设备
Manufacturer / 制造商
Keep dry / 保持干燥 / 怕雨
WEEE
S
N
Serial number
Permissible storage and transport
temperature and humidity
Storage/Transport
Operation
Permissible operating temperature
and humidity
Medical device
Distributor
Date of manufacture
Do not use outdoors
(indoor use only)
Single patient multiple use
PA
2020F213-44
中国GCC血压计计量证号
REF
Reference number
LOT
Batch number
I
ON
OFF
Dispose of packaging in an
environmentally friendly manner
70°C 95%
-25°C
Storage/Transport
10% 700hPa
1060hPa
10°C
40°C
95%
700hPa
1060hPa
Operating
10%
Importer
CAUTION /
注意!查阅随机文件
Type BF applied part /
BF型应用设备
Read the instructions for use /
请阅读说明书
Device in protection class 2 /
二类设备
Manufacturer / 制造商
Keep dry / 保持干燥 / 怕雨
WEEE
S
N
Serial number
Permissible storage and transport
temperature and humidity
Storage/Transport
Operation
Permissible operating temperature
and humidity
Medical device
Distributor
Date of manufacture
Do not use outdoors
(indoor use only)
Single patient multiple use
PA
2020F213-44
中国GCC血压计计量证号
REF
Reference number
LOT
Batch number
I
ON
OFF
Dispose of packaging in an
environmentally friendly manner
70°C 95%
-25°C
Storage/Transport
10% 700hPa
1060hPa
10°C
40°C
95%
700hPa
1060hPa
Operating
10%
Importer
CAUTION /
注意!查阅随机文件
Type BF applied part /
BF型应用设备
Read the instructions for use /
请阅读说明书
Device in protection class 2 /
二类设备
Manufacturer / 制造商
Keep dry / 保持干燥 / 怕雨
WEEE
S
N
Serial number
Permissible storage and transport
temperature and humidity
Storage/Transport
Operation
Permissible operating temperature
and humidity
Medical device
Distributor
Date of manufacture
Do not use outdoors
(indoor use only)
Single patient multiple use
PA
2020F213-44
中国GCC血压计计量证号
REF
Reference number
LOT
Batch number
I
ON
OFF
Dispose of packaging in an
environmentally friendly manner
70°C 95%
-25°C
Storage/Transport
10% 700hPa
1060hPa
10°C
40°C
95%
700hPa
1060hPa
Operating
10%
Importer
Recycling symbols/codes:
These are used to provide information about the
material and its proper use and recycling.
Protected against solid foreign objects 12.5
mm in diameter and larger, and directly
aected by water spray in any direction
without harmful eects.
Importer Unique device identication
IP25 IP25
0123
EC REP
Intended use
• The medisana inhaler IN 700 is intended for oral or nasal inhalati-
on therapy with medication aerosol (vibrating mesh technology) in
private households. The device may only be used by patients who
have thoroughly familiarised themselves with the function of the
device and with a liquid medication prescribed or recommended by
a doctor or pharmacist.
• The user needs to read and understand these instructions of use
and needs to understand the general machine operation.
• Always pay attention to the notes in the accompanying information
for the medication to be inhaled and follow the instuctions of your
doctor or pharmacist.
Applicable people
• The device can be used by adult or pediatric patients according to
the instructions in this manual.
0123
Safety Information
• Use the device only according to its intended purpose as specied in the instruction manual. The warranty will be invalidated
if used for purposes other than those for which it is intended.
• Any application other than the herewith described is improper and therefore considered dangerous. If the appliance is not
used according to the instructions, the user is liable for the safe operation of the unit.
• Like any medical device, this product may become unusable due to a power failure, dead battery or mechanical impact.
We recommend that you have a spare device available. Always observe basic safety precautions when using electrical
products. As with any electrical device, be especially careful around children.
• Only use medicines suitable for inhalation therapy in liquid form. Before starting therapy with the device, discuss the dura-
tion of use, dosage, frequency of use and choice of medication with your doctor or pharmacist.
• Never overll the tank of the device (max. 6 ml).
• DO NOT give this device to others. This product (including masks and mouthpiece) may only be used by one patient.
• Clean and disinfect the nebuliser head and accessories (mouthpiece or mask) according to the section “Cleaning and
disinfection”. Make sure that the nebuliser head and accessories have been properly cleaned and disinfected before use to
avoid possible contamination.
• The patient is the intended user of the device. Use of this device by children and persons requiring special assistance
requires adult supervision.
• To avoid the risk of entanglement and strangulation, keep the device and cables out of the reach of small children.
• For safety reasons, always disconnect the charging cable from the appliance in the following circumstances:
- if the mains adapter is damaged
- if a malfunction occurs during operation
- before cleaning the appliance
- immediately after use
- the product must not be put into operation if a fault is suspected.
• Do not store the product and accessories in a humid environment. Contamination and residual moisture favour the growth of
bacteria and increase the risk of infection.
• Do not use the device if the charging cable or other components are wet.
• DO NOT swallow small parts of the nebuliser.
• This device is intended for human use only.
• The mouthpiece and masks should be replaced with new parts approximately every 6 months, depending on the frequency
of use.
• DO NOT attempt to clean the inside of the unit with any foreign objects as this may damage the unit. Do not use any appli-
ance (e.g. dishwasher) to clean the appliance or its parts.
• Do not use the unit in an ambient temperature above 40 °C. For more information on the requirements for the operating
environment, see the chapter “Technical data”.
• Keep the unit away from direct sunlight, excessive heat or cold to avoid damage.
• DO NOT shake the unit during operation.
• Avoid any strong shock to the main unit and its components, e.g. by dropping it on the oor.
• DO NOT attempt to open, repair or modify the unit. Have repairs carried out only by authorised service centres.
• Always use only the accessories specied in these instructions for use.
• Before use, make sure that the device and its parts and components (e.g. mouthpiece and mask) are correctly assembled
according to these instructions for use. Follow local laws and recycling regulations regarding disposal or recycling of com-
ponents, batteries and packaging.
• The protection rating of this device is IPx5 - the device can be rinsed but not immersed in water or other liquids.
• DO NOT wash the adapter or charging cable. If any part of the unit comes into contact with liquid, dry it immediately.
• When connecting the unit to the mains, check the correct voltage at the sockets and make sure that the socket is not over-
loaded.
• Disconnect the unit from the power supply when the battery is fully charged and never leave a switched-on unit unattended.
• If the storage temperature of the unit has not been between 10-40 °C, please leave it in the operating environment for at
least 30 minutes before using it.
• Use the mouthpiece correctly according to the instructions for use and avoid the accumulation of medication on the tongue.
• Do not swallow the aerosol and make sure that the medicine reaches the aected area.
• For eective aerosol therapy, you should be calm and relaxed and sit still. The same applies if you are lying in bed for treat-
ment. Uncomfortable posture and restless breathing can lead to airway blockage.
• Make sure you maintain a correct posture during aerosol therapy. A steady and slow deep breath helps to inhale the aerosol
into the airways.
• Press the ON/OFF button when you want to stop the aerosol therapy. The device switches o automatically when the medi-
cine is used up.
• If there are many air bubbles between the medicine and the metal grid (mesh), the device cannot spray the aerosol. You can
switch o the unit rst. Then shake the nebuliser carefully. Finally, restart the unit.
• To ensure that the medicine can be nebulised normally, hold the unit vertically or tilt it slightly towards the metal grid. If an
operating unit is tilted so that the liquid does not come into contact with the nebuliser head, the unit will switch o after a delay
time of about 10 seconds (10 seconds applies to normal saline, other liquids may dier).
• Tilt the nebuliser slightly towards you until the solution is almost used up to ensure that the remaining solution that comes
into contact with the mesh is completely nebulised.
• Solutions with high viscosity may cause poor nebulisation or clogging of the mesh. In this case, switch o the unit and rinse
the accumulated solution on the mesh with distilled water.
• Be careful not to shake the unit during operation as this may cause the unit to malfunction, e.g. automatically shut down.
• Make sure that there is no liquid residue in the nebuliser head.
• Make sure that the nebuliser head has been thoroughly cleaned and disinfected after each use.
• Store the unit and accessories in a clean and safe place.
• Protect the unit from direct sunlight and shocks.
• Make sure that heaters and open res are far away from the storage place.
• Protect the unit from contact with corrosive liquids.
• Never dry the appliance and its accessories in a microwave oven.
• Never wrap a power cord around the appliance.
• Before each charging operation, check the integrity of the appliance, the cable and the plug used. If in doubt, do not connect
the appliance to the mains. Contact the customer service.
Items supplied and packaging
Please check rst of all that the unit is complete and is not damaged in any way. If in doubt, do not use the appliance. Send it
to a service point. The following parts are included:
- 1 medisana Inhaler IN 700 with 1 mouthpiece, 1 adult mask and 1 child mask
- 1 USB-C cable - 1 pouch - 1 Instruction manual
The packaging can be reused or recycled. Please dispose properly of any packaging material no longer required. If you
notice any transport damage during unpacking, please contact your dealer without delay.
WARNING
Danger of infection from contaminated nebulizer! Follow the hygiene regulations (e.g.
thoroughly washing the hands) before each use and make sure that the nebulizer has been
cleaned and disinfected before rst and after each use according to point „Cleaning and
disinfection“.
70°C 95%
-25°C
Storage/Transport
10% 700hPa
1060hPa
10°C
40°C
95%
700hPa
1060hPa
Operating
10%
PAP
20
Raccolta cartaRaccolta plastica
04
WARNING
Please ensure that the polythene packing is kept away from the reach of children!
Risk of suocation!
Charging the battery
Before using the device, it must be charged using the supplied USB-C cable
t
(approx. 2.5 hours are required to fully charge
an empty battery). To do this, connect the charging cable
t
to the charging cable connector
q
and connect it to a suitable
USB output or a suitable adapter (not included) and a power source. During charging, the power indicator
7
ashes blue -
when the unit is fully charged, the operating indicator 7 lights up blue constantly.
During battery operation and when the battery is suciently charged, the power indicator
7
lights up constantly green. If the
charge status becomes critical, the power indicator
7
lights up constantly yellow and the unit switches o automatically after
approx. 1 minute. In this case, connect the charging cable (connected to a power source) as a precaution (to completely nebu-
lise the remaining aerosol). However, we generally recommend charging the unit only when it is switched o.
The power indicator
7
Depending on the unit status, the power indicator
7
lights up as follows:
Constant green: Normal operating status (during use).
Constant yellow: Low battery charge. Unit switches o automatically after approx. 1 minute.
Constant blue: The battery is fully charged.
Flashing green: The unit is in cleaning mode.
Flashing yellow: No aerosol in the tank.
Flashing blue: Battery is being charged.
Preparing for inhalation
1. Clean and disinfect all parts as described under „Cleaning and disinfection“ to
avoid possible infection from contaminated parts.
2. Make sure that the nebuliser head unit
1
and base unit
9
are correctly assem-
bled. The assembly is done as shown opposite by inserting the nebuliser head unit
1
into the groove at the top of the base unit
9
and carefully pressing it together.
Note: If you want to separate the two parts again, press the disassembly button
0
and carefully pull the nebuliser head unit
1
from the base unit
9
.
3. Before using with a medication aerosol as described in the next chapter, you can also do a short test run of the unit with tap
water to see if correct operation is possible.
Perform inhalation
1. Fill the amount of inhalation solution instructed by the doctor (content minimum 2 ml and maximum 6 ml) into
the medicine cup
4
. To do this, you must open it while the device is still switched o, see illustration.
2. Close the medicine cup
4
and then put either the mouthpiece
w
or the adult mask
e
or the child mask
r
on the aerosol head
5
. The two masks must also be secured with the elastic band by putting them on around
the head.
3. Inhalation with the mouthpiece
w
: Sit in as upright a position as possible. Enclose the mouthpiece w
completely with your lips. Switch on the device with the ON/OFF switch
8
. The nebuliser produces a visible
mist (aerosol). Now breathe in slowly and deeply through your mouth and out again through your nose. If you
want to take a break, pause briey and take the mouthpiece
w
out of your mouth. Put it in your mouth again (lips enclosing
it tightly) and breathe in and out slowly again.
4. Inhalation with the mask
e
or
r
: With the help of the masks, the aerosol can be inhaled through the nose, which enables
application more for the upper respiratory tract. This application is found to be more pleasant, especially by children. Hold
the mask lightly pressed over the nose. The mask must enclose the nose tightly, but without pressing. Make sure that the
elastic headband is correctly placed around the head. Switch the unit on with the ON/OFF switch
8
. The nebuliser produces
a visible mist (aerosol). Now breathe in slowly and deeply through your nose. Then breathe out slowly through your mouth.
5. End inhalation: Switch o the device by pressing the ON/OFF switch
8
when you have nished inhaling, even if you have
not used up all the inhalation. Pour out any remaining inhalation. Do not use it again. Clean the device immediately after
each use (see also „Cleaning and disinfection“).
Cleaning and disinfection
Before and after each use, all parts of the device should be cleaned as described here and then additionally disinfected every
3 to 4 days to prevent the danger from growing microorganisms that increase the risk of infection.
Cleaning
1. Disconnect the USB-C cable
t
(if connected) from the unit. Remove the mouthpiece
w
or the adult mask
e
or the child
mask
r
from the nebuliser head unit
1
. Pour out any remaining contents of the medicine cup
4
and pour some tap water
into the medicine cup
4
, shake it gently and then pour out the water again.
2. Now add tap water to the medicine cup
4
again and then press and hold the ON/OFF switch
8
for approx. 5 seconds. The
the power indicator
7
starts ashing green and the unit switches to cleaning mode. After approx. 2 to 3 minutes you can
end this by pressing the ON/OFF switch
8
again. If you do not end it manually, the unit switches o automatically after 10
minutes. The automatic switch-o also occurs if the voltage is too low or there is no water in the medicine cup
4
.
3. Press the disassembly button
0
and carefully pull the nebuliser head unit
1
o the base unit
9
. Rinse the nebuliser head
unit
1
with distilled water, shake o excess water and then leave the nebuliser head unit
1
to dry on a dry surface. DO
NOT attempt to clean internal parts of the unit.
4. You can carefully wipe the base unit
9
with a clean, dry cloth.
5. The masks and the mouthpiece can be cleaned with a neutral detergent. For this purpose, put approx. 30°C warm water
and a little of the cleaning agent on a clean cloth and wash the parts with it for approx. 30 seconds. Then rinse thoroughly
with distilled water and leave to air dry.
6. If the inner mesh is very dirty, you can ll the medicine cup
4
with boiling water and a few drops of vinegar and start the
cleaning mode. If this does not remedy the situation, please contact the customer service.
7. Make sure that all parts are completely dry before storing or using the appliance again.
Disinfection
1. For disinfection, in addition to cleaning (see above), rinse the nebuliser head unit
1
and the accessories with distilled water
and then place the nebuliser head unit
1
in 75% ethyl alcohol for approx. 10 minutes.
2. Boil the accessories (mouthpiece and masks) in water for about 5 minutes.
3. Afterwards, please rinse nebuliser head unit
1
and the accessories again with distilled water.
4. Shake o excess water and allow the parts to air dry completely on a clean surface.
5. Make sure that all parts are completely dry before storing or using the appliance again.
Storage and maintenance
• Before storage, please make sure that there is no residual liquid in the nebuliser head unit
1
.
• Store the unit and accessories in a clean, dry and safe place.
• Protect the unit from direct sunlight and shocks.
• Remember to store the unit away from heat sources or open re.
• Protect the unit from contact with corrosive liquids.
• Ensure compliance with the storage conditions specied in the technical data.
WARNING
Never immerse the base unit
9
in water or other liquids or rinse it with them!
Troubleshooting
Problem: No or only low nebulisation performance.
Possible reason: The nebuliser head unit
1
is not assembled correctly or is held at an angle, the mesh or the attachment
parts are dirty, the battery power is insucient or there is a defect.
Solution: Follow the instructions for cleaning and disinfection and handling. If you cannot solve the problem, contact the
customer service.
Problem: The power indicator
7
does not light up and/or unit does not work.
Possible reason: Problem with the power supply or the battery voltage.
Solution: Fully charge the battery and try to switch the unit on again. If you cannot solve the problem, contact the customer
service.
Problem: The power indicator
7
lights up, but the device does not work.
Possible reason: Problem with the power supply or battery voltage, electrodes 6 or parts of the unit are dirty or the unit is
tilted too much.
Solution: Fully charge the battery and try turning the device on again. Separate the nebulizer head unit
1
and base unit
9
and carefully clean the electrodes
6
with a cotton swab. Follow the cleaning and disinfection steps. Be careful not to tilt the
device too much during use. If you cannot solve the problem, contact customer service.
Problem: The device switches itself o during inhalation.
Possible reason: Too much inclination or too restless posture.
Solution: Do not tilt the device too much and try to sit as still as possible. Strong vibrations can result in insucient mesh-liquid
contact. If you cannot solve the problem, contact customer service.
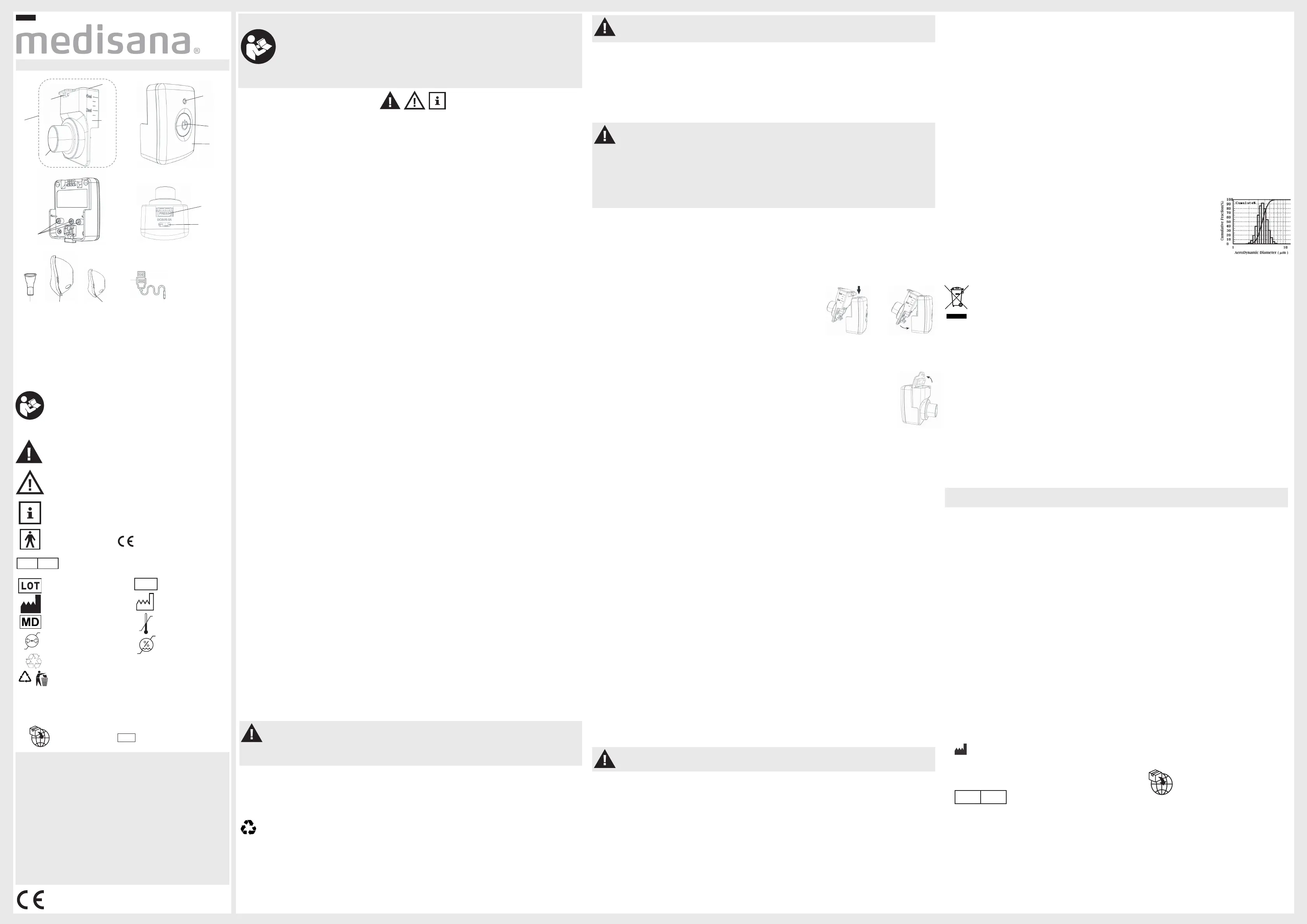
Directives / norms
This inhalation device complies with the requirements of Directive 93/42/EEC (EC Medical Devices Direc-
tive) and EN 13544-1: 2007 + A1: 2009 „Respiratory therapy devices - Part 1: Nebuliser systems and their
components“, Annex CC.3 Use of a multistage cascade impactor to measure particle size. Test conditions:
Temperature 24±2°C, humidity 45%-75% R.H., solution NaF 2.5% (M/V). Electromagnetic compatibility: The
instrument complies with the requirements of standard EN 60601-1-2 for electromagnetic compatibility. Details
of this measurement data can be found in the separate supplement.
Classication
• Devices that are not suitable for use near ammable mixtures.
• Type BF applied part. The mask or mouthpiece you put on are application parts.
• Devices with internal power supply (without adapter).
• The degree of protection against water ingress is IPX5.
Disposal
This product must not be disposed of together with domestic waste. All users are obliged to hand in all electrical or
electronic devices, regardless of whether or not they contain toxic substances, at a municipal or commercial collection
point so that they can be disposed of in an environmentally acceptable manner. Consult your municipal authority or
your dealer for information about disposal.
Technical specications
Name and model: medisana Inhaler IN 700, model: NB-813B
Power supply: DC 5V, 0.5A
Rated Power: ≤3W
Ultrasonic Frequency: Approx. 110 kHz
Nebulizing Rate: ≥0,2ml/min
Max./Min. Liquid Volume: 6ml/2ml
MMAD / FPF: <5μm / ≥60%
Noise level: <50 dB
Fully Charged Battery Life: >1 hour
Time to Fully Charge The Battery: <2,5 hours
Built-in Lithium Battery information: Battery capacity: 350 mAh, Normal voltage: 3.7 V
Expected service life: 500 cycles
Automatic Shutdown without medicine: YES
Cleaning Mode: YES
Expected Serviced Life: Main unit:3 years, Nebulizer head:180 hours, Accessories:6 months
Operating Conditions: +10°C - +40°C, rel. humidity 30%~85%, Air pressure 800 hPa~1060 hPa
Storage and Transport Conditions: -10°C - +40°C, rel. humidity 10 - 85 %, Air pressure 500 hPa - 1060 hPa
Pollution Degree: Degree 2
Voltage Category: II
Altitudes: ≤2000M
Weight / Dimensions LxWxH approx.: 45g / 54 x 36 x 44 mm (Main unit)
Article no. / EAN number: 54545 / 40 15588 54545 0
The current version of this instruction manual can be found under www.medisana.com
Warranty and repair terms
Your statutory warranty rights are not restricted by our guarantee below. Please contact your dealer or the service centre in
case of a claim under the warranty. If you have to return the unit, please enclose a copy of your receipt and state what the
defect is. The following warranty terms apply:
1. The warranty period for medisana products is three years from date of purchase. In case of a warranty claim, the date
of purchase has to be proven by means of the sales receipt or invoice.
2. Defects in material or workmanship will be removed free of charge within the warranty period.
3. Repairs under warranty do not extend the warranty period either for the unit or for the replacement parts.
4. The following is excluded under the warranty:
a. All damage which has arisen due to improper treatment, e.g. nonobservance of the user instructions.
b. All damage which is due to repairs or tampering by the customer or unauthorised third parties.
c. Damage which has arisen during transport from the manufacturer to the consumer or during transport to the service
centre.
d. Spare parts which are subject to normal wear and tear.
5. Liability for direct or indirect consequential losses caused by the unit are excluded even if the damage to the unit is
accepted as a warranty claim.
The service centre address is shown on the attached leaet.
HONSUN (NANTONG) Co., Ltd.
Add: No.8, Tongxing Road, Economic&Technical
Development Area, Nantong City, Jiangsu, P.R.CHINA
TEL: +86-21-63056696, e-Mail: market@lordmed.com
imported by
medisana GmbH
Carl-Schurz-Str. 2
41460 NEUSS
GERMANY
Shanghai International Holding Corp. GmbH
(EUROPE)
Add: Eiestrasse 80, 20537 Hamburg, GERMANY
EC REP
Read the instruction manual carefully before using this device,
especially the safety instructions, and keep the instruction manu-
al for future use. Should you give this device to another person, it
is vital that you also pass on these instructions for use.
GB IMPORTANT INFORMATION! RETAIN FOR FUTURE USE!
GB Instruction manual - Inhaler IN 700
WARNING
• Increased risk of leakage, avoid contact with skin, eyes and mucous membranes! In case of contact with
battery acid, rinse the aected areas immediately with plenty of clean water and consult a doctor imme-
diately!
• The built-in battery cannot be removed. The unit does not contain any parts that can be serviced by the
user.
• Do not disassemble the battery or the unit, do not throw it into a re and protect it from excessive heat!
• Before rst use, fully charge the battery according to these instructions for use.
• If you do not use the device for a longer period of time, fully charge the battery approx. every 6 months
to optimise the service life. Before charging, run the unit with some distilled water for a few minutes to
discharge the battery. The optimal charging ambient temperature should be between 10°Cand 40°C.
In accordance with our policy of continual product improvement, we reserve the right to make technical
and optical changes without notice.
70°C 95%
-25°C
Storage/Transport
10% 700hPa
1060hPa
10°C
40°C
95%
700hPa
1060hPa
Operating
10%
PAP
20
Raccolta cartaRaccolta plastica
04
UDI
medisana GmbH
Carl-Schurz-Str.2, 41460 NEUSS
GERMANY
www.medisana.de
PAP
20
21
Bekijk gratis de handleiding van Medisana IN 700, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Medisana |
| Model | IN 700 |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 6587 MB |







