Medel Air Plus handleiding
Handleiding
Je bekijkt pagina 34 van 64
34
Medication flow
rate Approx. 0.3 ml/min
Filling volume
Nasal douche
Min. 2 ml
Max. 10 ml
Sound pressure Approx. 52 dBA
(acc. to DIN EN 13544-1 section
26)
Power supply 230 V~; 50 Hz; 230 VA
UK: 240 V~; 50 Hz; 240 VA
Saudi Arabia: 220 V~; 60 Hz; 220
VA
Expected service
life 1000 h
Operating
conditions
Temperature: +10°C to +40°C
Relative humidity: 10% to 95%
Ambient pressure: 700 to 1060
hPa
Storage and
transportation
conditions
Temperature: 0°C to +60°C
Relative humidity: 10% to 95%
Ambient pressure: 500 to 1060
hPa
Aerosol proper
-
ties
1) Flow: 5.3 l/min
2) Aerosol delivery: 0.11 ml
3) Aerosol delivery rate: 0.07 ml/
min
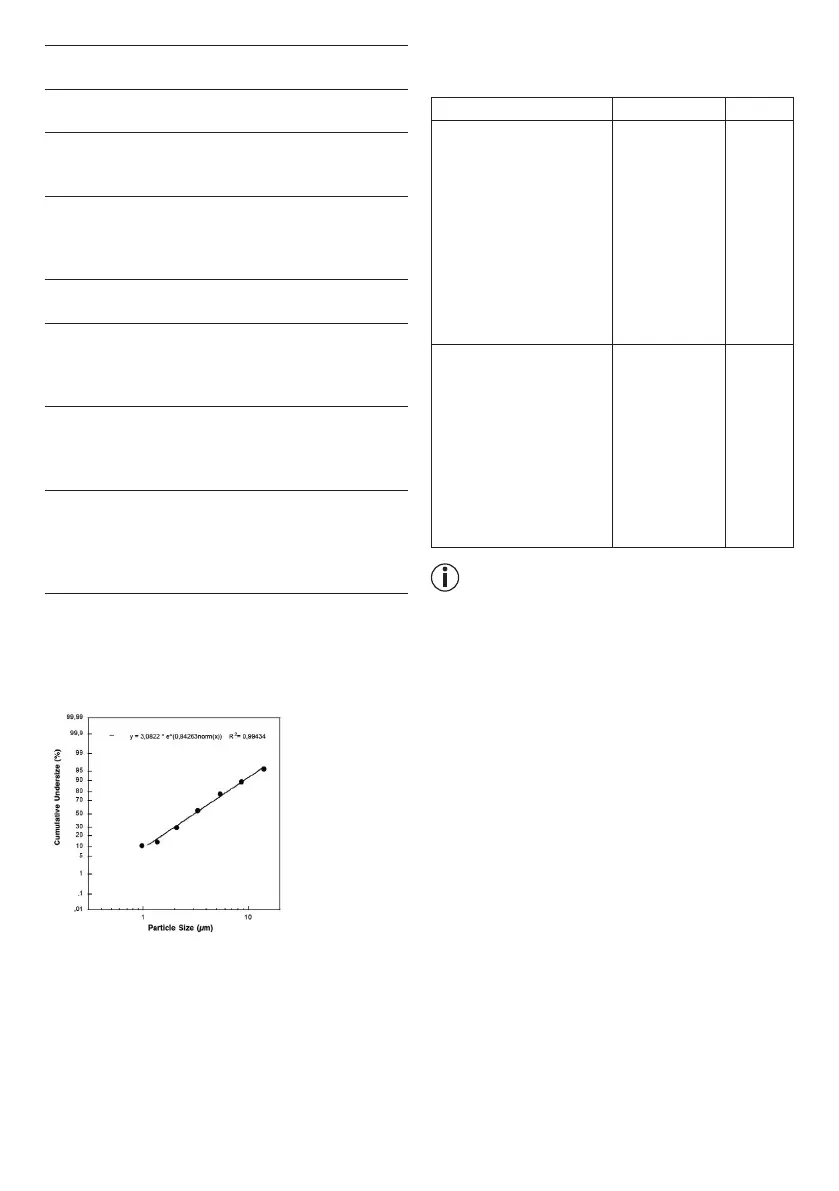
4) Particle size (MMAD): 3.07 µm
The serial number is located on the device or in the
battery compartment.
Subject to technical changes.
Particle size diagram
Cumulative undersize (%)
Measurements were performed using a sodium fluoride
solution with a “Next Generation Impactor” (NGI).
The diagram may therefore not be applicable to sus-
pensions or highly viscous medications. You can obtain
more detailed information from the manufacturer of
your medication.
12. Replacement parts and wea-
ring parts
Designation Material REF
Year pack AIR PLUS
Standard contains:
Mouthpiece
Nosepiece
Adult mask
Children’s mask
Nebulizerer
Compressed air hose
Filter
PP/Silicone
PP/Silicone
PVC/Alu
-
minum
PVC/Alu
-
minum
PP/Silicone
PVC
PU
603.33
Yearpack AIR PLUS Kids
contains:
Mouthpiece
Silicon children mask
Silicone baby mask
Angled fitting
Nebulizer
Compressed air hose
Filter
PP/Silicone
Silicone/PP
Silicone/PP
PP
PP/Silicone
PVC
PU
603.34
Note
If the device is not used according to the instructions
specified, perfect functionality cannot be guaranteed!
We reserve the right to make technical changes to
improve and develop the product. This device and
its accessories comply with the European standards
EN60601-1, EN60601-1-2 (CISPR 11, IEC61000-
3-2, IEC61000-3-3, IEC61000-4-2, IEC61000-4-
3, IEC61000-4-4, EC61000-4-5, IEC61000-4-6,
IEC61000-4-7, IEC61000-4-8, IEC61000-4-11) and
EN13544-1 and are subject to particular precautions
with regard to electromagnetic compatibility. This de-
vice meets the requirements of European Directive
93/42/EEC for medical products, as well as those of the
Medizinproduktegesetz (German Medical Devices Act).
NOTES ON ELECTROMAGNETIC COMPATIBILITY
•
The device is suitable for use in all environments
listed in these instructions for use, including domes-
tic environments.
•
The use of the device may be limited in the presence
of electromagnetic disturbances. This could result in
issues such as error messages or the failure of the
display/device.
•
Avoid using this device directly next to other devices
or stacked on top of other devices, as this could lead
to faulty operation. If, however, it is necessary to use
the device in the manner stated, this device as well
Bekijk gratis de handleiding van Medel Air Plus, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Medel |
| Model | Air Plus |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 8099 MB |


