Beurer EM 89 handleiding
Handleiding
Je bekijkt pagina 39 van 228
39
Pulse duration 50–450 µs
Pulse frequency 1–150 Hz
Output voltage max. 100 Vpp (at 500 Ohm)
Output current max. 200 mApp (at 500 Ohm)
Voltage supply Lithium-ion battery, 4000mAh, 3.7V
Treatment time adjustable from 5 to 100 minutes
Intensity Adjustable from 0 to 50
Maximum temperature of
the heat settings
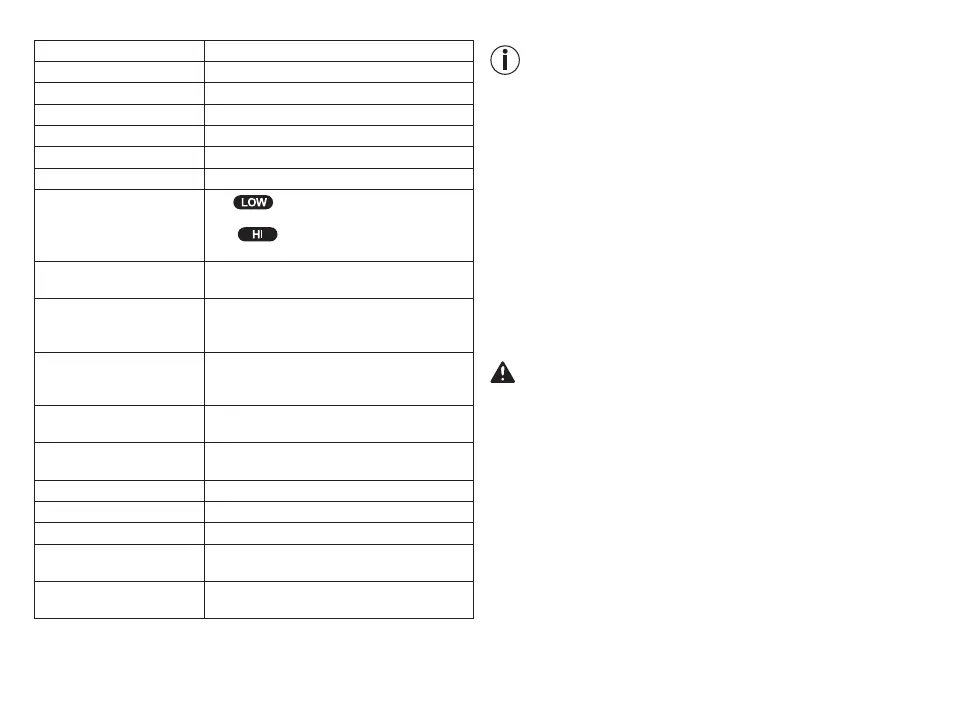
low
(41°C at an ambient temperature
of 25°C.);
high (43°C at an ambient temperature
of 25°C.)
Electrodes used Silver electrodes with carbon coating
40 x 40mm
Mains adapter to be used Output: 5V, 2A
Material number: See “Replacement parts
and wearing parts” section
Operating conditions 5°C to 40°C (41°F to 104°F), with a relative
air humidity of 15-90% and air pressure of
70-106 kPa
Storage conditions -25 to 70°C (-13°F to 158°F), with a relative
air humidity of ≤90%
Transport conditions: -25°C to 70 °C (-13°F to 158°F), with a
relative air humidity of ≤90%
Dimensions Approx. 142 x 159 x 53 mm
Weight Approx. 341 g
Altitude limit for use 3,000 m
Maximum tolerated
atmospheric pressure
700 hPa to 1060 hPa
Expected service life of the
device
Information on the service life of the product
can be found on the homepage
The serial number is located on the device.
The device is maintenance-free. Inspections and calibration are not necessary.
If the device is not used according to the specifications, it may not
work correctly!
We reserve the right to make technical changes to improve and develop the
product.
This device complies with the European standard EN 60601-1-2 (Group 1,
Class B, in compliance with IEC 61000-4-2, IEC 61000-4-3, IEC 61000-4-8 and
IEC 61000-4-39) and is subject to special precautionary measures with regard
to the electromagnetic compatibility. Please note that portable and mobile HF
communication systems may interfere with this device.
More details can be requested from the stated Customer Service address or
found at the end of the instructions for use.
For this device, a functional test and instruction in accordance with the German
Medical Devices Operator Ordinance (MPBetreibV) is not required. It is also not
necessary to carry out safety checks in accordance with the German Medical
Devices Operator Ordinance (MPBetreibV).
Notes on electromagnetic compatibility
WARNING!
• The device is suitable for use in all environments listed in these instructions
for use, including domestic environments.
• The device may not be fully usable in the presence of electromagnetic dis-
turbances. This could result in issues such as error messages or the failure
of the display/device.
• Avoid using this device directly next to other devices or stacked on top of
other devices, as this could lead to faulty operation. If, however, it is neces-
sary to use the device in the manner outlined above, this device as well as
the other devices must be monitored to ensure they are working properly.
• The use of components other than those specified or provided by the man-
ufacturer of this device can lead to an increase in electromagnetic emis-
sions or a decrease in the device’s resistance to electromagnetic interfer-
ence; this can result in faulty operation.
• Keep portable RF communication devices (including peripheral equipment
such as antenna cables or external antennas) at least 30 cm away from all
device parts, including all cables included in delivery. Failure to comply with
the above could impair the performance of the device.
Bekijk gratis de handleiding van Beurer EM 89, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Beurer |
| Model | EM 89 |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 23250 MB |







