Thames & Kosmos Pepper Mint in the Fantastic Underwater Science Voyage handleiding
Handleiding
Je bekijkt pagina 19 van 36
WHY CAN’T WATER BE
COMPRESSED?
You’ve almost certainly noticed before that very cold
water below 0° Celsius freezes and turns to ice, or that if
you boil water (over 100° Celsius), it evaporates and
disappears. This is referred to as “states of maer.” Heat
plays a very special part in these changes from solid to
liquid to gas. The warmer water gets, the more its
molecules bounce around because they have more
energy. With ice, the molecules are frozen into a grid-
like laice structure. In water, this rigid structure comes
apart, and the molecules start moving about. In steam,
they shoot around all over the place, so much so that
they’re barely even connected to each other any more.
When water is in liquid form, the molecules move
about, however because of the forces of araction and
repulsion that exist between the individual molecules,
they stay at a set distance from each other. This is why
you can’t compress the water in your syringe.
?
WHAT’S
HAPPENING
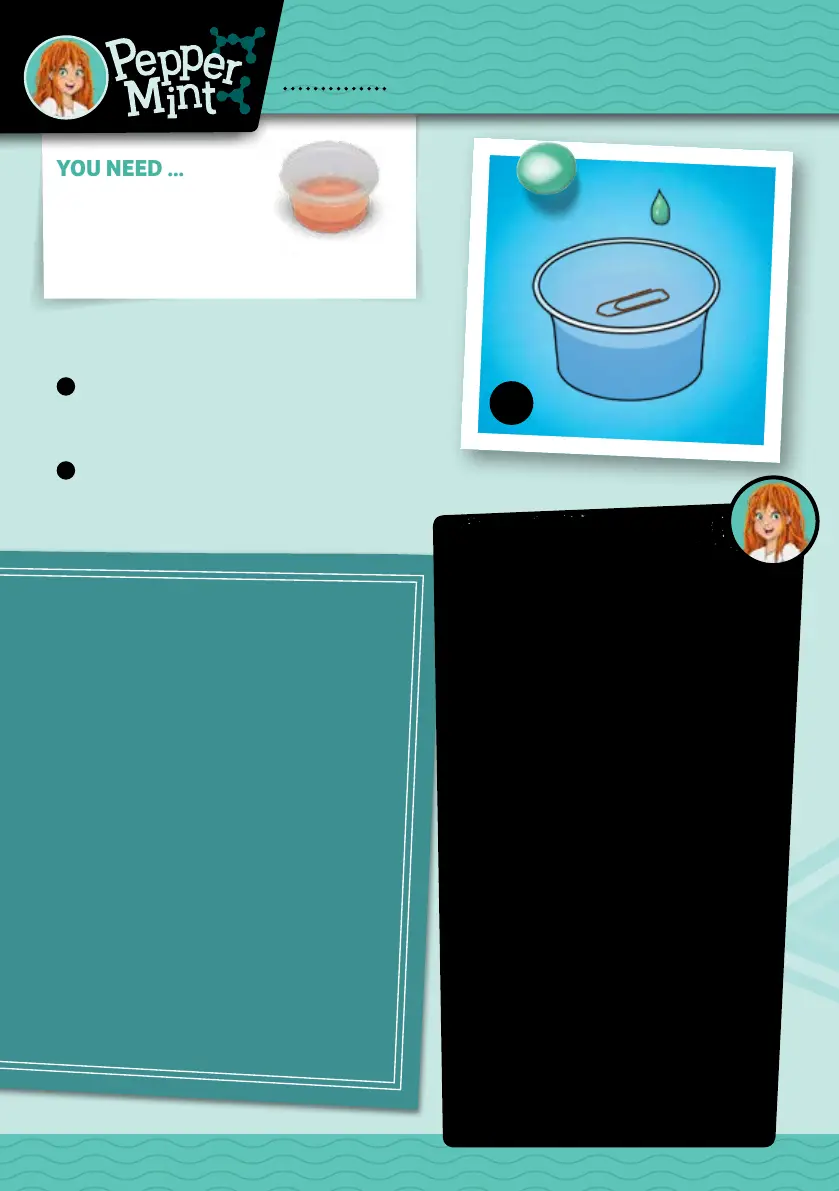
The paper clip floats on the surface — until you
add soap and disturb the “surface tension” of the
water. Then it sinks to the
boom. Water
consists of a great number of molecules that
aract and repel each other. Ifa molecule is
surrounded by other water
molecules, these
interactions balance each other out. If a water
molecule comes in contact with air, because
it’s at the surface, the forces of araction can
no longer be balanced out, and the molecule is
drawn inward into the liquid. Water
tries to
form the smallest
possible surface area in
relation to the substance that borders it. This
is why water forms round droplets, and why
it has asurface tension that allows small
creatures, like water striders for example, to
walk on it. The soap is made up of molecules
that push between water molecules on the
surface, disturbing the water’s surface
tension.
HERE’S HOW!
1
Fill two thirds of your tub with tap water.
Carefully place a paper clip or pin onto the
surface of the water.
2
Drop a lile dishwashing liquid into the water.
1
YOU NEED ...
Tub
You will also need: tap water,
paper clips, soap or dishwashing
liquid, coins
16
Nature of Water
AND THE
Bekijk gratis de handleiding van Thames & Kosmos Pepper Mint in the Fantastic Underwater Science Voyage, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Thames & Kosmos |
| Model | Pepper Mint in the Fantastic Underwater Science Voyage |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 24761 MB |







