Thames & Kosmos Ooze Labs: Chemistry Station handleiding
Handleiding
Je bekijkt pagina 14 van 20
1
2
3
1
WHAT’S HAPPENING
The vinegar and the baking soda react
together. A gas, namely carbon dioxide, is
formed. This gas takes up much more volume
than the vinegar and the baking soda did, so
it expands and fills up the balloon. This gas
is quite harmless. You are already familiar
with it from the fizz in a bottle of soda, as
well as other experiments in this kit.
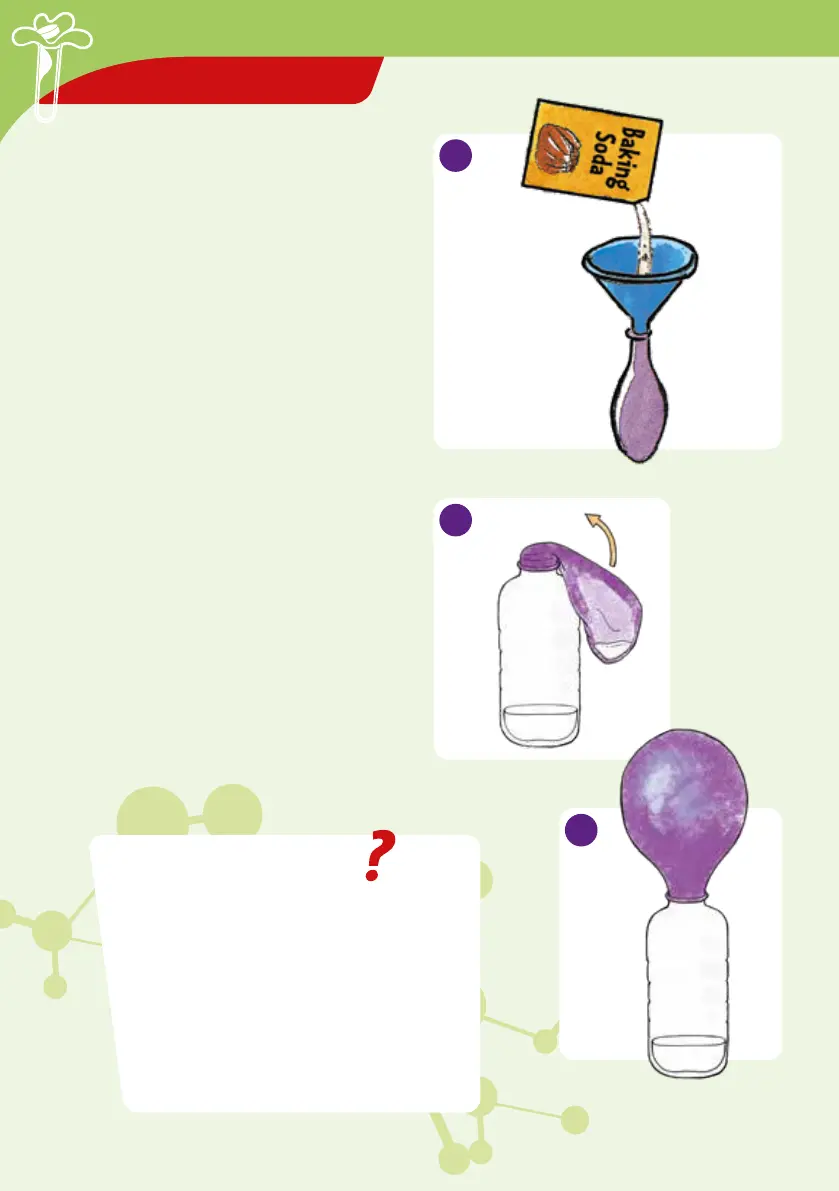
Self-inflating
balloon
A balloon can also be used to capture gasses
coming from a chemical reaction.
YOU WILL NEED
Funnel, measuring spoon, small measuring
cup, rubber balloon, baking soda, vinegar,
plastic water bottle
HERE’S HOW
1. Put four spoonfuls of baking soda into an
uninflated, pre-stretched balloon. You may
need to have a helper hold the balloon.
2. Pour 5 ml of vinegar into the plastic water
bottle. Put the mouth of the balloon over
the bottle, and lift the balloon so that the
baking soda falls down into the vinegar.
3. Observe. A reaction will occur and carbon
dioxide gas will form. Hold the mouth of
the balloon tightly on the bottle so it does
not come off.
› › › EXPERIMENT 15
12
Bekijk gratis de handleiding van Thames & Kosmos Ooze Labs: Chemistry Station, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Thames & Kosmos |
| Model | Ooze Labs: Chemistry Station |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 6140 MB |







