Thames & Kosmos Kids First: Crystals, Rocks & Minerals handleiding
Handleiding
Je bekijkt pagina 13 van 36
WHAT’S HAPPENING
When a solid substance — such as the alum in this case —
dissolves, water pushes between its smallest component parts
(molecules) and releases them from their compounds. These
building blocks are then floating around individually in the
water. Since they are very tiny, you can’t see them. With many
substances, their ability to dissolve increases with temperature
(a well-known exception is table salt). A solution that can’t
dissolve anything further at a certain temperature
is called saturated. When the solution cools off it
becomes supersaturated, and the excess alum
separates out in the form of crystals. This cooling
method provides crystals very rapidly.
Overnig ht
TIP !
If the resulting crystals are not big enough, you can put them
back into the solution, warm it up again, and then wrap the
cooling jar with a thick towel so that it cools more slowly.
Larger crystals will form when the solution cools more slowly.
Cooling method
YOU WILL NEED
› Large measuring cup
› Double-ended measuring spoon
› g Potassium aluminum sulfate
(alum powder)
› Wooden spatula
› Display box
› Distilled water
› Pot of hot water (40-50 °C, 100-120 °F)
› Paper towels
› 2 Empty glass jelly jars with lids
› Sheet of paper
› Pencil
HERE’S HOW
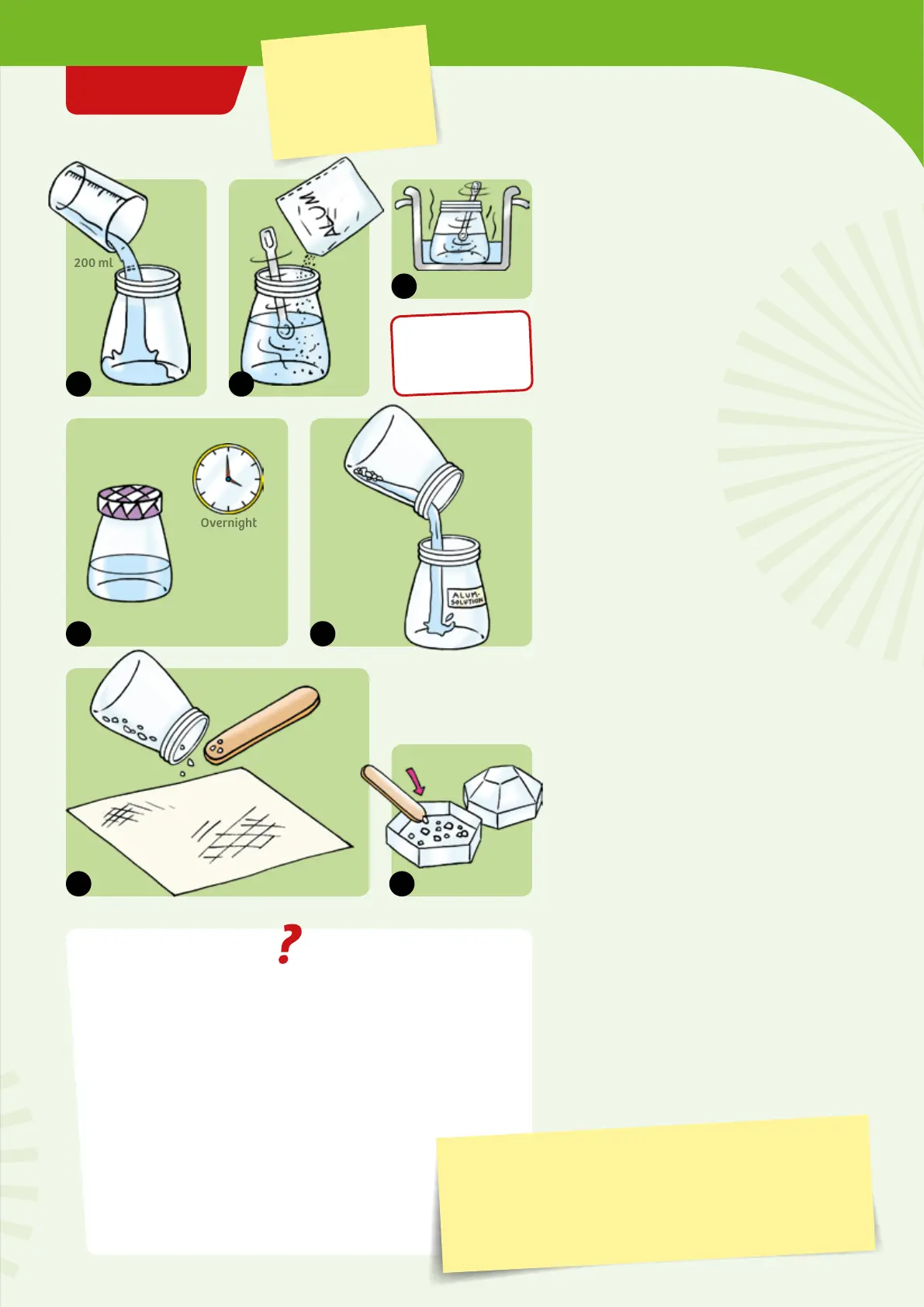
1. Use the measuring cup to measure 200 ml
of distilled water and pour it into the empty
jelly jar.
2. Add the alum powder and stir. Even after
you have stirred for a long time, a large
part of the alum will remain undissolved at
the bottom.
3. Set the jar into the pot of hot water and
resume stirring. Now everything dissolves.
4. Screw the lid onto the jar, and place it in a
quiet place for a few hours. A lot of crystals
will form.
5. Pour the solution into the second jar. Label
the jar and save it for the next experiment.
6. Shake the small crystals onto a paper
towel with the help of the wooden spatula.
Let them dry and select a few large and
pretty crystals.
7. Put them in the display box and close it
well (see page 9). You will need them later
when you make your geode.
1
3
5
6 7
4
Be careful when
handling hot water!
Wear protective
goggles!
2
200 ml
Important:
For all of the
experiments, wear
your safety goggles!
EXPERIMENT 1
Homemade Crystals
Bekijk gratis de handleiding van Thames & Kosmos Kids First: Crystals, Rocks & Minerals, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Thames & Kosmos |
| Model | Kids First: Crystals, Rocks & Minerals |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 30537 MB |







