Thames & Kosmos Glowing Chemistry handleiding
Handleiding
Je bekijkt pagina 42 van 52
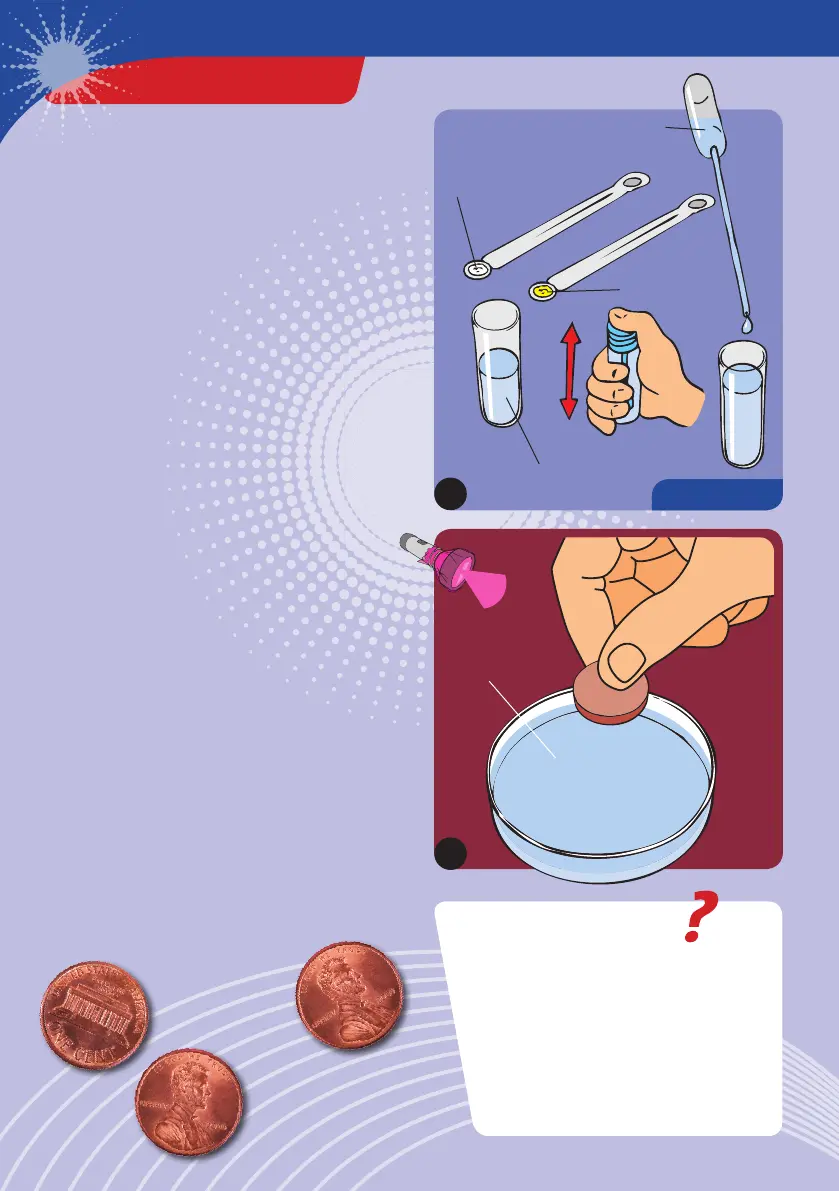
EXPERIMENT 18
1
3
Light Solution
Light
Solution
WHAT’S HAPPENING
The coin starts to glow slightly — the
dirtier it was, the brighter the glow.
The coin is coated with copper, and
copper compounds are also capable
of setting off the luminol light
reaction.
40
Penny in a blue sea
Potassium hexacyanoferrate(III) is not the only
thing that can make the luminol start to shine.
YOU WILL NEED
› Safety goggles
› Luminol
› Sodium carbonate
› Test tube
› Double-headed measuring spoon
› Pipette
› Petri dish
› Hydrogen peroxide
› Penny (one-cent coin)
HERE’S HOW
1. Prepare some light solution (luminol-
hydrogen peroxide-sodium carbonate
solution) as in the last experiment.
2. Pour the light solution into the Petri dish.
3. Darken the room completely, turn on the red
light, and wait a few minutes for your eyes
to get used to the dim light. Then place the
penny in the Petri dish and immediately turn
off the red light.
After the experiment, remove the coin from
the dish with the double-headed measuring
spoon and wash it off.
Sodium carbonate
Luminol
Hydrogen peroxide
Water
Bekijk gratis de handleiding van Thames & Kosmos Glowing Chemistry, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Thames & Kosmos |
| Model | Glowing Chemistry |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 26917 MB |







