Thames & Kosmos Global Water Quality handleiding
Handleiding
Je bekijkt pagina 19 van 20
EXPERIMENT 8 Sparkling water sparkles
4
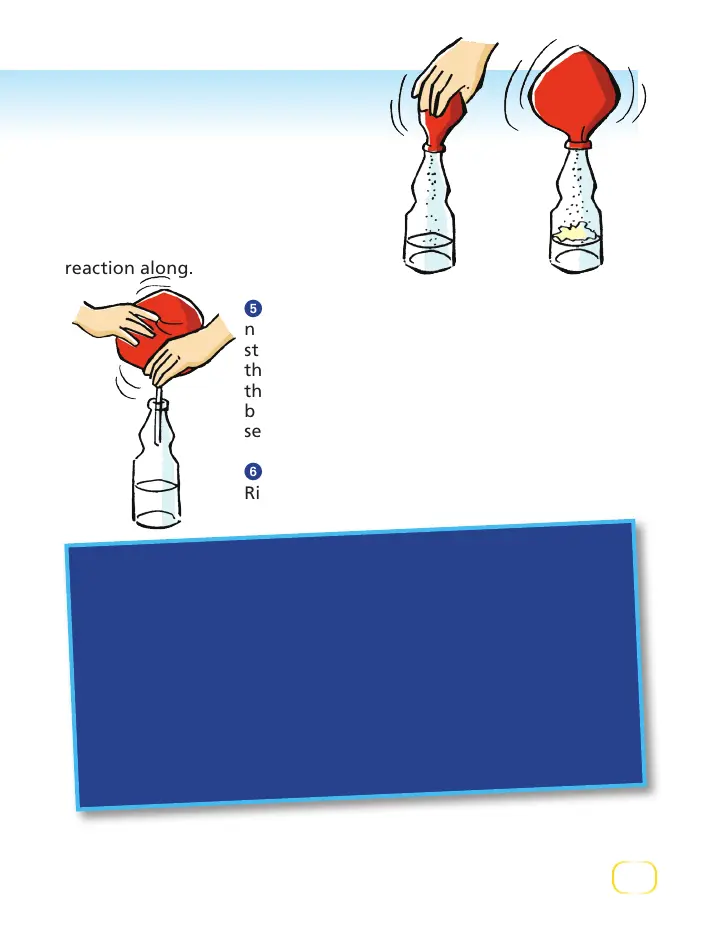
Lift up on the balloon so that
all the baking soda falls into the
bottle. It will react with the vinegar
and produce a lot of carbon dioxide
gas, which will inflate the balloon.
Shake the bottle a little to help the
reaction along.
5
Carefully remove the balloon, being sure
not to let the gas escape. With the help of the
straw, force the gas into the second bottle so
that it collects on top of the water. Remove
the balloon and quickly twist the cap onto the
bottle. Shake the bottle vigorously for a few
seconds. What happens?
6
You can pour the liquid down the drain.
Rinse the sink with water afterwards.
Explanation:
There is a lot of carbon dioxide chemically bonded in the baking soda
powder. An acid, in this case vinegar, releases it.
When the carbon dioxide is transferred into the bottle with the
water, it pushes out the air from inside the bottle. After you shake it,
a portion of the carbon dioxide has disappeared. It has dissolved in
the water to form carbonic acid. That means that there is some gas
missing in the bottle, which collapses in on itself.
Unfortunately, this gas, which we humans produce in large quantities
every day, also dissolves in the world’s oceans, where it turns into
carbonic acid and harms ocean creatures such as coral and shellfish.
Water
Vinegar
More Tests!
Now test the pH value of the water!
17
Global Water Quality Manual 2013.indd 17 2/7/13 10:22 AM
Bekijk gratis de handleiding van Thames & Kosmos Global Water Quality, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Thames & Kosmos |
| Model | Global Water Quality |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 3490 MB |







