Thames & Kosmos Creative Cosmetics Lab handleiding
Handleiding
Je bekijkt pagina 28 van 36
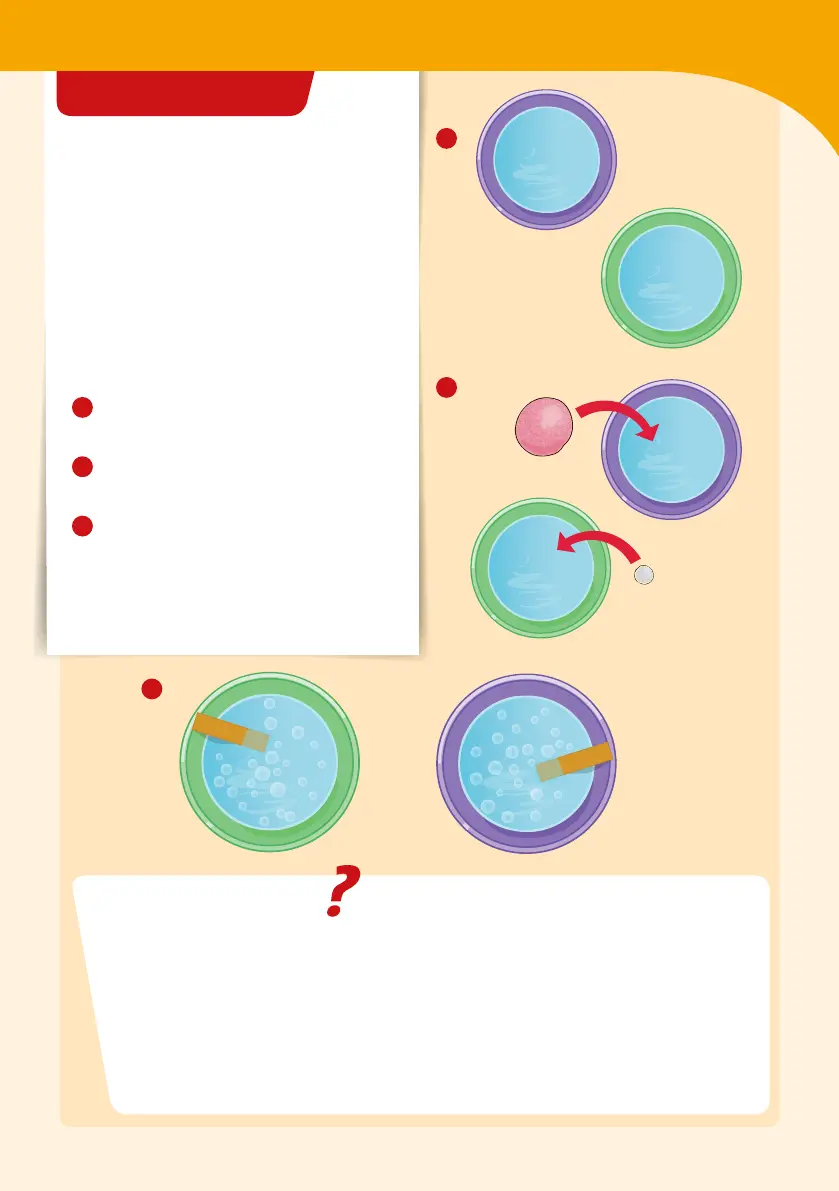
HERE’S HOW
1
Fill each bowl with enough water
to cover a bath bomb.
2
Place a bath bomb in each of the
bowls of water at the same time.
3
Compare the amount of bubbling
that the two bath bombs produce.
Then dip a pH strip into each of the
bowls and compare the pH of the
solutions.
Fizzy bath bomb
experiment
YOU WILL NEED
One bath bomb each from Experiments
9 and 10, two pH strips, two bowls,
water
EXPERIMENT 11
WHAT’S HAPPENING
The bubbling of the bath bombs is caused by a chemical reaction that is taking place in
the bowls. Both bowls exhibit a fizzing reaction. In the first version of the bath bomb, the
sodium hydrogen carbonate (also known as sodium bicarbonate), which is a base, is
reacting with the tartaric acid. The products of this reaction are carbon dioxide gas (the
bubbles), water, and a chemical called sodium tartrate. In the second, the sodium
hydrogen carbonate is reacting with the potassium dihydrogen phosphate, which is an
acid (like tartaric acid). The products of this reaction are also carbon dioxide and
water, but in this case a chemical called hydrogen phosphate.
1
2
3
25
Acids and Bases
Bekijk gratis de handleiding van Thames & Kosmos Creative Cosmetics Lab, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Thames & Kosmos |
| Model | Creative Cosmetics Lab |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 10798 MB |







