Thames & Kosmos Chem C1000 handleiding
Handleiding
Je bekijkt pagina 17 van 19
15
EXPERIMENT
03
EXPERIMENT
04
!
!
?
Denatured alcohol: Highly ammable liquid and vapor. Keep away
from heat/sparks/open ames/hot surfaces. – No smoking. – Keep
container tightly closed.
For sodium carbonate and citric acid, note the “Hazardous
substances and mixtures” information on pp. 7 – 8.
Question 1. The safety closures are designed to prevent small
children from opening the vials. What features make this a safety
closure, and why?*
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
WARNINGWARNINGWARNINGWARNINGWARNING
WARNINGWARNINGWARNINGWARNINGWARNING
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
DANGER
WARNING
WARNINGWARNINGWARNINGWARNINGWARNING
WARNINGWARNINGWARNINGWARNINGWARNING
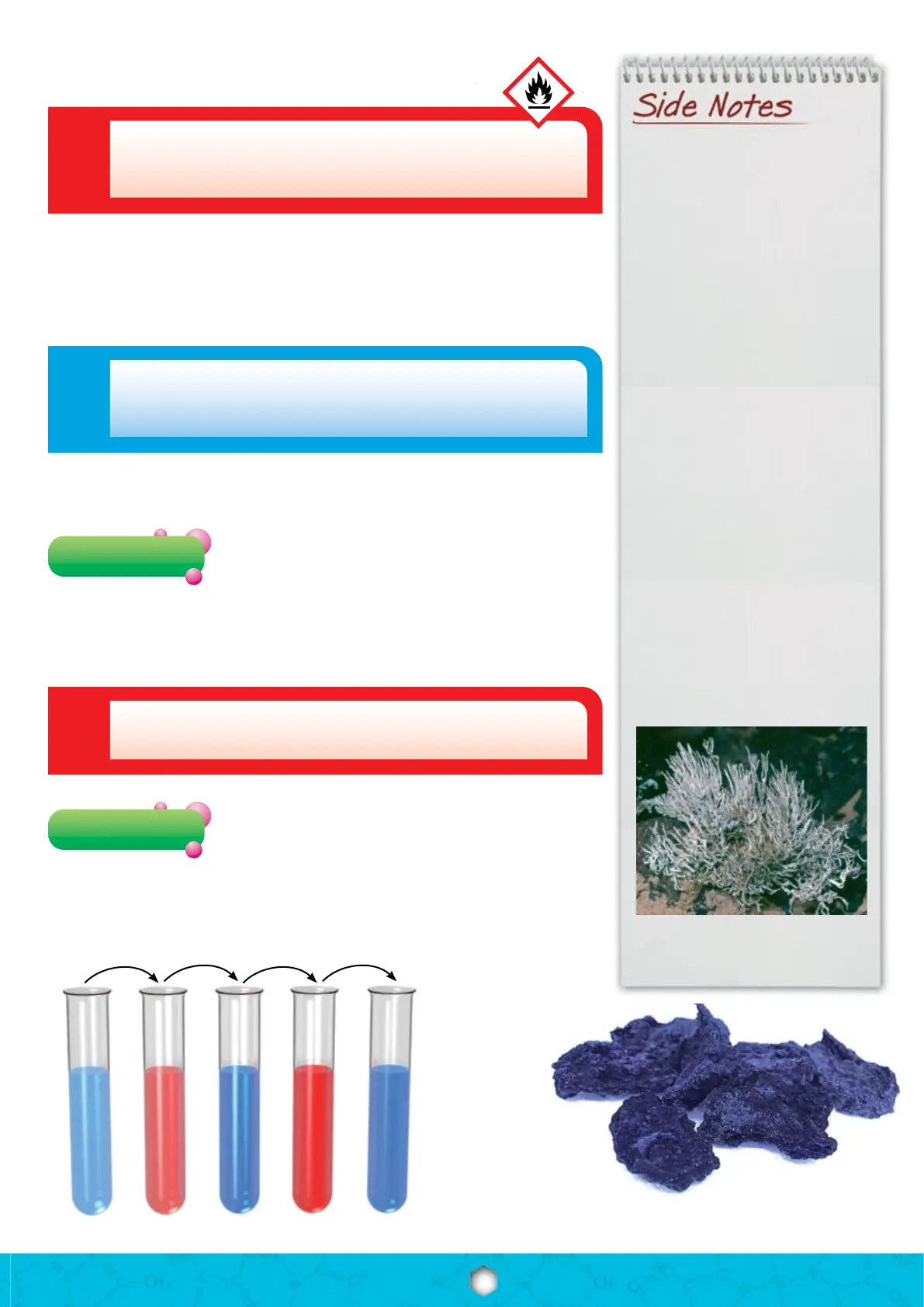
Litmus is a plant product with a limited shelf life.
The denatured alcohol acts as a preservative.
Blue here,
red there
+ Vinegar + Sodium + Citric + Sodium
carbonate acid carbonate
The optical brightener of the
Netherlands
Litmus is a mixture of plant materials
that is obtained above all from lichens
such as Roccella montagnei shown
below. The Dutch once used the dye
for “bluing” their laundry. The blue
covers the yellow tint in textiles and
thus produces the “whitest of whites.”
Today, the “optical brighteners”
contained in detergents — products of
chemical ingenuity — do the trick.
The name comes from the Netherlands,
too: lakmoes, a Dutch word whose
origin linguists are not completely in
agreement about. There is pretty much
a consensus about the second syllable,
moes, meaning puree, pulp, mush.
Whereas some think that lak means
paint (hence “colored pulp”), others
say that hidden in lak is the Old Dutch
word lêken, meaning to drip. Both
interpretations are consistent with the
preparation process. The lichens were
ground with water between millstones
into a colored pulp that was allowed to
ferment and drain. The dried deep-blue
mass was then ground into powder.
The lichen Roccella montagnei, from
which litmus powder is obtained
(photo: Prof. Dr. V. Wirth, Karlsruhe).
Now place the safety closure with dropper insert onto the vial and close it (by
turning the closure clockwise). The illustration on the previous page shows how to
close and open the safety closure by turning and pressing it at the same time.
When relling (recommended due to the limited shelf life), the dripper
insert must rst be removed. Have an adult help you with this if necessary. Okay,
now the litmus solution is ready to use.
Blue here, red there
Additional material: White vinegar (wine vinegar)
To a test tube with 8 mL of water, add 3 drops of litmus
solution. The solution is light blue. Pour a little vinegar
into a test tube and drip it into the litmus solution using a pipette. The rst drops
already cause the color to turn bright red. You will need this solution for the next
experiment.
Regarding Experiments 4 – 8:
Add a spoon tip of sodium carbonate to the red solution
from the previous experiment. Rock the tube gently back
and forth: The solution turns blue again, this time a bit darker than the aqueous
(watery) solution in Experiment 3. Don’t throw this solution away, either!
Pressed litmus (© and photo: chemie-
master.de)
Bekijk gratis de handleiding van Thames & Kosmos Chem C1000, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Thames & Kosmos |
| Model | Chem C1000 |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 11336 MB |







