Thames & Kosmos Candy Chemistry handleiding
Handleiding
Je bekijkt pagina 25 van 52
A solution is a mixture of substances in which
the particles of one substance are evenly
mixed with the particles of the other
substance. When you put a spoonful of sugar
into water and stir it around, it seems to
vanish into the water. In actuality, you’re
creating a solution of sugar and water. The
sugar dissolves into the water. But, we don’t
call sugar-water a compound, because the
sugar and water molecules are not bonded
together, and can be separated by physical
means.
Solutions can be made of different
combinations of phases of matter. A solid can
be dissolved in a liquid, gas, or another solid.
Liquids and gases can also be dissolved in
each of the three phases of matter. Saltwater
is an example of a solid dissolved in a liquid,
and Earth’s atmosphere is an example of
many gases (like oxygen) dissolved in another
gas (nitrogen).
A solution consists of a solute, the substance
that is dissolved, and a solvent, the
substance that dissolves the solute. If
something can be dissolved, we say that it’s
soluble. If something cannot, we say that it’s
insoluble. The solubility of a solution is a
measure of the amount of solute than can go
into the solvent at a given temperature.
Water is a particularly good solvent, because
water molecules are like little magnets with
positively and negatively charged sides.
Molecules with positively and negatively
charged sides are referred to as polar. The
charged sides help pull molecules of solute
apart to dissolve them. Some molecules, like
sugar, dissolve into water without the
molecule actually breaking apart, while
others, like salt, break apart into their
component atoms. The former is called a
molecular solution, and the latter is called an
ionic solution.
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
H
H
H
H
H
H
H
H
H
H
H
H
O
O
O
H
O
C
C
C
C
C
C
C
C
C
C
O
O
O
H
H
H
C
O
H
H
H
C
O
O
O
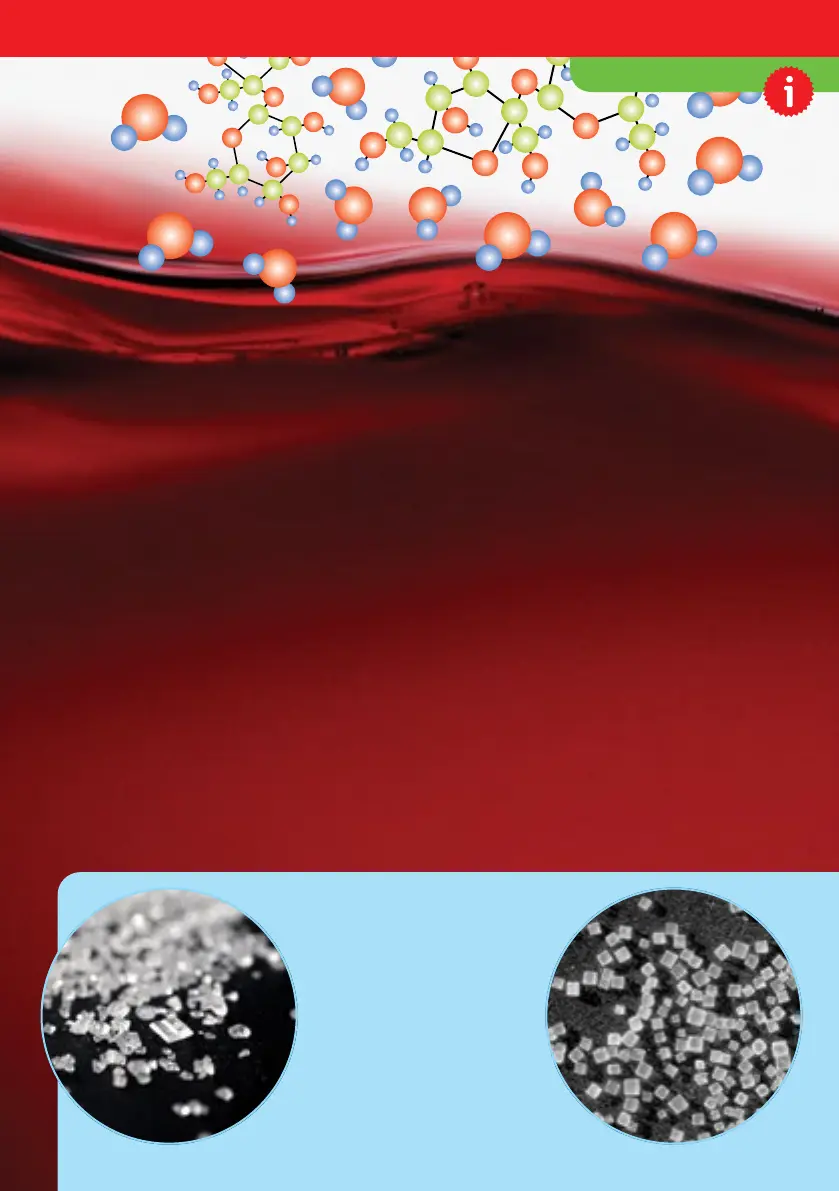
SOLUTIONS
SUGAR VS. SALT
Sugar and salt molecules
both form crystals, but if you
look closely with a
magnifying glass, you will
see that salt crystals are
little cubes, while sugar
crystals are oblong with
slanted angles on their sides.
SUGAR
SALT
CHECK IT OUT
Sugar Solutions and Crystals | 23
Bekijk gratis de handleiding van Thames & Kosmos Candy Chemistry, stel vragen en lees de antwoorden op veelvoorkomende problemen, of gebruik onze assistent om sneller informatie in de handleiding te vinden of uitleg te krijgen over specifieke functies.
Productinformatie
| Merk | Thames & Kosmos |
| Model | Candy Chemistry |
| Categorie | Niet gecategoriseerd |
| Taal | Nederlands |
| Grootte | 30778 MB |







